Abstract
Mitochondrial diseases are associated with defects of adenosine triphosphate production and energy supply to organs as a result of dysfunctions of the mitochondrial respiratory chain. Biallelic mutations in the YARS2 gene encoding mitochondrial tyrosyl-tRNA synthetase cause myopathy, lactic acidosis, and sideroblastic anemia 2 (MLASA2), a type of mitochondrial disease. Here, we report a consanguineous Turkish family with two siblings showing severe metabolic decompensation including recurrent hypoglycemia, lactic acidosis, and transfusion-dependent anemia. Using whole-exome sequencing of the proband and his parents, we identified a novel YARS2 mutation (c.1303A>G, p.Ser435Gly) that was homozygous in the patient and heterozygous in his parents. This mutation is located at the ribosomal protein S4-like domain of the gene, while other reported YARS2 mutations are all within the catalytic domain. Interestingly, the proband showed more severe symptoms and an earlier onset than previously reported patients, suggesting the functional importance of the S4-like domain in tyrosyl-tRNA synthetase.
Similar content being viewed by others
Main
Aminoacyl-tRNA synthetases (ARSs) are essential enzymes that attach specific amino acids to the corresponding tRNAs (aminoacylation). Among a total of 36 human ARSs , YARS (tyrosyl-tRNA synthetase) and YARS2 (tyrosyl-tRNA synthetase 2; mitochondrial ARSs are nominally numbered ‘2’) catalyze the binding of tyrosine to their cognate cytoplasmic and mitochondrial tRNAs, respectively.1 YARS2 is encoded by the nuclear gene YARS2 (NM_001040436.2) at 12p11.21. ARSs do not complement each other. Mutations in 11 of 17 mitochondrial ARS genes cause a wide variety of diseases according to PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and the Human Genome Mutation Database professional (https://portal.biobase-international.com/hgmd/pro/start.php).2 For example, biallelic mutations in DARS2, RARS2, FARS2, and AARS2 cause leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation (MIM#611105), pontocerebellar hypoplasia, type 6 (MIM#611523), combined oxidative phosphorylation deficiency 14 (MIM#614946) showing fatal epileptic encephalopathy, and combined oxidative phosphorylation deficiency 8 (MIM#614096) presenting with lethal infantile cardiomyopathy, respectively.3, 4, 5, 6 YARS2 defects also cause loss of mitochondrial tyrosyl-tRNA (mt-tRNATyr) leading to the failure of protein production in mitochondria.1, 7 YARS2 mutations cause myopathy, lactic acidosis, and sideroblastic anemia 2 (MLASA2, MIM#613561),8, 9, 10 which is an autosomal recessive disorder characterized by relatively mild symptoms of oxidative phosphorylation defects including progressive muscle weakness and sideroblastic anemia.8, 9, 10 To our knowledge, only four families with YARS2 mutations have thus far been reported.8, 9, 10
The proband (II-4) is the fourth child of healthy Turkish parents who are first cousins (Figure 1a). He was born by normal delivery at 39 weeks of gestation with a birth weight of 2900 g. The pregnancy and birth history were uneventful. On the 4th day of life, he showed poor feeding, tachypnea (80 breaths/min), metabolic acidosis (pH 7.14, PCO2 26.7 mm Hg, HCO3− 5.1 mmol l−1, base excess 18.6 mmol l−1), and hyperlactacidemia (lactate 3.74 mmol l−1) while carnitine, acylcarnitine, and quantitative amino acid analysis of plasma and urine were normal. Following a few weeks without any symptoms after the discharge, he suffered the rapid progression of normocytic anemia and recurrent metabolic decompensation including lactic acidosis, ketosis, and hyperammonemia (Supplementary Table 1). At 7 weeks of age, red blood cells were transfused due to the rapidly progressive anemia (Supplementary Table 2). At 2 months of age, he showed axial hypotonia. His ophthalmologic examination at this age was normal, although a brain magnetic resonance imaging scan showed thinning of the corpus callosum with normal progress of myelination. An echo cardiogram revealed hypertrophy of the interventricular septum and left ventricle. The presence of proteinuria and hypercalciuria may indicate proximal renal tubulopathy (Supplementary Table 3). The glomerular filtration rate and serum levels of calcium, phosphate and vitamin D were within normal range. Although 4OH-phenyllactate and 4OH-phenylpyruvate were elevated, the transaminase level was within normal range. He was admitted to the hospital total of five times because of episodic metabolic decompensation, while there were no obvious triggering factors like infection.
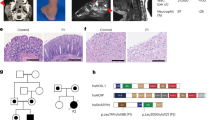
Genetic analysis of the YARS mutation in this pedigree. (a) Pedigree tree of the affected family and mutation segregation. (b) Electropherograms of the YARS2 mutation (c.1303A>G). The mutated base is marked by a square. Evolutionary conservation is shown at the bottom. MT, mutant allele; WT, wild type allele (c) Schema of YARS2 protein with mutational localization. The patient’s mutation is colored in red below the diagram of the protein, while previously reported mutations (p.Gly46Asp and p.Phe52Leu) are in black. MTS, mitochondrial target sequence; N-core and C-core, N and C part of the catalytic domain, respectively; CP1, connective peptide; α-ACB, α-helical anticodon-binding domain; S4-like, ribosomal protein S4-like protein; a.a., amino acid.
During the episodic metabolic decompensation, serum lactate, pyruvate, the lactate/pyruvate ratio, ketone bodies, Krebs cycle intermediates, ammonia and creatine kinase levels were all increased. Plasma amino acid analysis revealed remarkably high alanine levels (Supplementary Table 1). He was treated with supportive therapies including the intravenous infusion of glucose (10 mg kg−1 min−1) and sodium bicarbonate according to the calculation of HCO3- deficit (0.5 × body weight (kg) × 24 h-serum HCO3− (mEq/l)), and responded promptly within one hour after starting the therapy. As a defect of the mitochondrial respiratory chain (MRC) was suspected, he was treated with sodium dichloroacetate (50 mg kg−1 day−1), coenzyme Q10, carnitine, biotin, and riboflavin. Unfortunately, he died at the age of 3 months from a cardiopulmonary arrest that occurred during a metabolic decompensation. The other affected sib (II-2) died at the age of 2 days following a similar clinical course to the proband. Unfortunately, detailed clinical information about this patient was unavailable.
To identify the genetic cause of their condition, we performed whole-exome sequencing on the proband (II-4) and his parents (I-1 and I-2) as described in Supplementary Methods. This study was approved by the institutional review board of Yokohama City University School of Medicine. As two of the four children from healthy parents were affected, we hypothesized that the disorder was an autosomal recessive disease and focused on homozygous variants of the WES data. After excluding synonymous variants and variants registered in dbSNP137, ESP6500, and our in-house database (exome data of 408 individuals), five homozygous variants remained (Supplementary Tables 4, 5). As four variants predicted as ‘benign’ by PolyPhen-211 and/or ‘polymorphism’ by MutationTaster12 were excluded, only one homozygous missense mutation, c.1303A>G, p.Ser435Gly, in exon 5 of the YARS2 gene was highlighted (Supplementary Table 5), which is known to cause MLASA2. Sanger sequencing revealed that only proband had homozygous YARS2 mutation while the parents and unaffected sibs had a heterozygous one (Figures 1a and b). HomozygosityMapper13 (http://www.homozygositymapper.org/) confirmed that this mutation was located within a 3.5 Mb homozygous stretch.
Interestingly, two affected patients in this study showed more severe clinical phenotypes than previously reported patients with MLASA2,8, 9, 10 including recurrent metabolic decompensation, proximal renal tubulopathy, and brain abnormalities which are rarely seen in MLASA2 patients,8, 9, 14 (Table 1, Supplementary Table 6). Early onset severe progressive anemia necessitating a blood transfusion was common to both our patient and the previously reported MLASA2 patients; this is most likely a result of the severe metabolic impairment of erythropoiesis. Unfortunately, we were unable to perform a bone marrow aspirate and a peripheral blood smear test to determine whether our patients had sideroblastic anemia because of their rapid deterioration.
Human YARS2 has a catalytic domain and an anticodon-binding region (Figure 1c). This anticodon-binding region consists of an α-helical anticodon-binding domain and a ribosomal protein S4-like domain (S4-like domain).15 The S4-like domain is essential to recognize tRNA, and is evolutionarily well conserved from eubacteria to humans.16, 17 The mutation in our patient was located in the S4-like domain whereas all other previously reported YARS2 mutations were in the catalytic domain (Figure 1c).8, 9, 10 The difference of mutation location may explain the clinical differences among the patients. Furthermore, the mutated amino acid serine 435 is highly conserved from frog to human (Figure 1b). The change from a hydrophilic serine to a hydrophobic glycine residue might alter the protein static structure and impair the physiological function of YARS2.18 Thus, an abnormal S4-like domain would impair the tyrosylation of mitochondrial tRNA resulted in MRC dysfunction.
In this study, WES technique appears to be the powerful method, especially for suspected mitochondrial diseases showing various clinical phenotypes. This is because the involvement of many mutant genes in MRC disorders hampers regular Sanger sequencing of candidate genes,19, 20 and biopsies and enzymological analysis of affected organs may be difficult because of the severity and rapid progression of the disease.
References
Antonellis, A. & Green, E. D. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu. Rev. Genomics Hum. Genet. 9, 87–107 (2008).
Stenson, P. D., Mort, M., Ball, E. V., Shaw, K., Phillips, A. D. & Cooper, D. N. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum. Genet. (e-pub ahead of print 28 Sep 2013; doi: 10.1007/s00439-013-1358-4).
Scheper, G. C., van der Klok, T., van Andel, R. J., van Berkel, C. G., Sissler, M., Smet, J. et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat. Genet. 39, 534–539 (2007).
Edvardson, S., Shaag, A., Kolesnikova, O., Gomori, J. M., Tarassov, I., Einbinder, T. et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am. J. Hum. Genet. 81, 857–862 (2007).
Gotz, A., Tyynismaa, H., Euro, L., Ellonen, P., Hyotylainen, T., Ojala, T. et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 88, 635–642 (2011).
Elo, J. M., Yadavalli, S. S., Euro, L., Isohanni, P., Gotz, A., Carroll, C. J. et al. Mitochondrial phenylalanyl-tRNA synthetase mutations underlie fatal infantile Alpers encephalopathy. Hum. Mol. Genet. 21, 4521–4529 (2012).
DiMauro, S. & Schon, E. A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Sasarman, F., Nishimura, T., Thiffault, I. & Shoubridge, E. A. A novel mutation in YARS2 causes myopathy with lactic acidosis and sideroblastic anemia. Hum. Mutat. 33, 1201–1206 (2012).
Riley, L. G., Cooper, S., Hickey, P., Rudinger-Thirion, J., McKenzie, M., Compton, A. et al. Mutation of the mitochondrial tyrosyl-tRNA synthetase gene, YARS2, causes myopathy, lactic acidosis, and sideroblastic anemia—MLASA syndrome. Am. J. Hum. Genet. 87, 52–59 (2010).
Shahni, R., Wedatilake, Y., Cleary, M. A., Lindley, K. J., Sibson, K. R. & Rahman, S. A distinct mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) phenotype associates with YARS2 mutations. Am. J. Med. Genet. A 161, 2334–2338 (2013).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Seelow, D., Schuelke, M., Hildebrandt, F. & Nurnberg, P. HomozygosityMapper—an interactive approach to homozygosity mapping. Nucleic Acids Res. 37, W593–599 (2009).
Inbal, A., Avissar, N., Shaklai, M., Kuritzky, A., Schejter, A., Ben-David, E. et al. Myopathy, lactic acidosis, and sideroblastic anemia: a new syndrome. Am. J. Med. Genet. 55, 372–378 (1995).
Bonnefond, L., Fender, A., Rudinger-Thirion, J., Giege, R., Florentz, C. & Sissler, M. Toward the full set of human mitochondrial aminoacyl-tRNA synthetases: characterization of AspRS and TyrRS. Biochemistry 44, 4805–4816 (2005).
Yaremchuk, A., Kriklivyi, I., Tukalo, M. & Cusack, S. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 21, 3829–3840 (2002).
Guijarro, J. I., Pintar, A., Prochnicka-Chalufour, A., Guez, V., Gilquin, B., Bedouelle, H. et al. Structure and dynamics of the anticodon arm binding domain of Bacillus stearothermophilus Tyrosyl-tRNA synthetase. Structure 10, 311–317 (2002).
Grantham, R. Amino acid difference formula to help explain protein evolution. Science 185, 862–864 (1974).
Tucker, E. J., Compton, A. G. & Thorburn, D. R. Recent advances in the genetics of mitochondrial encephalopathies. Curr. Neurol. Neurosci. Rep. 10, 277–285 (2010).
Bernier, F. P., Boneh, A., Dennett, X., Chow, C. W., Cleary, M. A. & Thorburn, D. R. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 59, 1406–1411 (2002).
Acknowledgements
We thank the patient and his family for participating in this work. We also thank Ms S Sugimoto and K Takabe for technical assistance. This work was supported by research grants from the Ministry of Health, Labour and Welfare (N. Matsumoto, N. Miyake), the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Ministry of Education, Culture, Sports, Science and Technology (N. Matsumoto), the Strategic Research Program for Brain Sciences (N. Matsumoto), a Grant-in-Aid for Scientific Research on Innovative Areas-(Transcription cycle)-from the Ministry of Education, Culture, Sports, Science and Technology of Japan (N. Matsumoto), a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (H. Saitsu, N. Matsumoto, N. Miyake), the Takeda Science Foundation (N. Matsumoto, N. Miyake), and the Hayashi Memorial Foundation for Female Natural Scientists (N. Miyake).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Nakajima, J., Eminoglu, T., Vatansever, G. et al. A novel homozygous YARS2 mutation causes severe myopathy, lactic acidosis, and sideroblastic anemia 2. J Hum Genet 59, 229–232 (2014). https://doi.org/10.1038/jhg.2013.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2013.143
Keywords
This article is cited by
-
Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia
International Journal of Hematology (2018)
-
Mutations in methionyl-tRNA synthetase gene in a Chinese family with interstitial lung and liver disease, postnatal growth failure and anemia
Journal of Human Genetics (2017)
-
Clinical and molecular study in a long-surviving patient with MLASA syndrome due to novel PUS1 mutations
neurogenetics (2016)
-
A peep into mitochondrial disorder: multifaceted from mitochondrial DNA mutations to nuclear gene modulation
Protein & Cell (2015)
-
Familial schwannomatosis with a germline mutation of SMARCB1 in Japan
Brain Tumor Pathology (2015)