Abstract
Background/Objective:
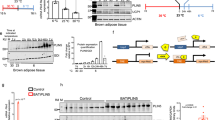
White-to-brown adipose tissue remodeling (browning) in response to different stimuli constitutes an active research area for obesity treatment. The emergence in traditional white adipose tissue (WAT) depots of multilocular adipocytes that express uncoupling protein 1 (UCP1) and resemble brown adipocytes, the so called 'brite' adipocytes, could contribute to increased energy expenditure. In rodents, obesogenic stimuli such as the intake of hyperlipidic diets can increase brown adipose tissue (BAT) thermogenic capacity and contribute to maintaining body weight. The aim of this study was to investigate the potential of two different hyperlipidic diets, a commercial high-fat (HF) diet and a highly palatable cafeteria (CAF) diet, to induce WAT browning.
Methods:
We analyzed gene expression of a wide number of brown/brite adipocyte markers in different WAT depots, in BAT and in peripheral blood mononuclear cells (PBMCs) increasingly being used in nutrition studies as a potential source of biomarkers of physiological effects. We also performed morphological analysis of adipose tissue.
Results:
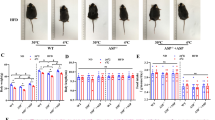
Both HF diets studied were able to increase the expression of the markers studied in WAT in a depot-specific manner, as well as in BAT; some of these changes were also reflected in PBMCs. This increased browning capacity was translated into the appearance of UCP1- and CIDE-A (cell death-inducing DFFA-like effector A)-positive brite adipocytes in retroperitoneal WAT. Administration of the CAF diet, associated with higher adiposity, produced the strongest impact on the parameters studied while its withdrawal restored basal conditions.
Conclusions:
Acquisition of brown adipocyte features in WAT could evidence an adaptation to try to counteract increased adiposity due to the intake of HF diets. Additionally, PBMCs could constitute an interesting easily obtainable material to assess the effect of nutritional interventions on browning capacity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hill JO, Wyatt HR, Peters JC . Energy balance and obesity. Circulation 2012; 126: 126–132.
Jequier E . Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci 2002; 967: 379–388.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Palou A, Pico C, Bonet ML, Oliver P . The uncoupling protein, thermogenin. Int J Biochem Cell Biol 1998; 30: 7–11.
Nedergaard J, Bengtsson T, Cannon B . Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531.
Whittle AJ, Lopez M, Vidal-Puig A . Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med 2011; 17: 405–411.
Lo KA, Sun L . Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci Rep 2013; 33: 711–719.
Bonet ML, Oliver P, Palou A . Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 2013; 1831: 969–985.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J . Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285: 7153–7164.
Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S . Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279: C670–C681.
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298: E1244–E1253.
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7: e49452.
Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006; 36: 485–501.
Dziedzic B, Szemraj J, Bartkowiak J, Walczewska A . Various dietary fats differentially change the gene expression of neuropeptides involved in body weight regulation in rats. J Neuroendocrinol 2007; 19: 364–373.
Brooks SL, Rothwell NJ, Stock MJ, Goodbody AE, Trayhurn P . Increased proton conductance pathway in brown adipose tissue mitochondria of rats exhibiting diet-induced thermogenesis. Nature 1980; 286: 274–276.
Rothwell NJ, Stock MJ . A role for brown adipose tissue in diet-induced thermogenesis. Obes Res 1997; 5: 650–656.
Fromme T, Klingenspor M . Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1–R8.
Caimari A, Oliver P, Keijer J, Palou A . Peripheral blood mononuclear cells as a model to study the response of energy homeostasis-related genes to acute changes in feeding conditions. Omics 2010; 14: 129–141.
Oliver P, Reynes B, Caimari A, Palou A . Peripheral blood mononuclear cells: a potential source of homeostatic imbalance markers associated with obesity development. Pflugers Arch 2013; 465: 459–468.
Reynes B, Garcia-Ruiz E, Diaz-Rua R, Palou A, Oliver P . Reversion to a control diet is able to restore body weight and to recover altered metabolic parameters in adult rats fed on a cafeteria diet. Food Res Int 2014; 64: 839–848.
Caimari A, Oliver P, Rodenburg W, Keijer J, Palou A . Slc27a2 expression in peripheral blood mononuclear cells as a molecular marker for overweight development. Int J Obes (Lond) 2010; 34: 831–839.
Ribot J, Rodriguez AM, Rodriguez E, Palou A . Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity (Silver Spring) 2008; 16: 723–730.
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 2009; 37: e45.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Caimari A, Oliver P, Rodenburg W, Keijer J, Palou A . Feeding conditions control the expression of genes involved in sterol metabolism in peripheral blood mononuclear cells of normoweight and diet-induced (cafeteria) obese rats. J Nutr Biochem 2010; 21: 1127–1133.
Hsu SM, Raine L, Fanger H . Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981; 29: 577–580.
Venegas V, Wang J, Dimmock D, Wong LJ . Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet 2011; Chapter 19, Unit 19.7.
Phillips NR, Sprouse ML, Roby RK . Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: a multiplex real-time PCR assay. Sci Rep 2014; 4: 3887.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 2013; 8: e60563.
Hojna S, Jordan MD, Kollias H, Pausova Z . High-fat diet induces emergence of brown-like adipocytes in white adipose tissue of spontaneously hypertensive rats. Hypertens Res 2012; 35: 279–286.
Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 2003; 35: 49–56.
Wu L, Zhou L, Chen C, Gong J, Xu L, Ye J et al. Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci China Life Sci 2014; 57: 107–116.
Campbell CT, Kolesar JE, Kaufman BA . Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta 2012; 1819: 921–929.
Gburcik V, Cawthorn WP, Nedergaard J, Timmons JA, Cannon B . An essential role for Tbx15 in the differentiation of brown and "brite" but not white adipocytes. Am J Physiol Endocrinol Metab 2012; 303: E1053–E1060.
Boss O, Farmer SR . Recruitment of brown adipose tissue as a therapy for obesity-associated diseases. Front Endocrinol (Lausanne) 2012; 3: 14.
Oliver P, Sanchez J, Caimari A, Miralles O, Pico C, Palou A . The intake of a high-fat diet triggers higher brown adipose tissue UCP1 levels in male rats but not in females. Genes Nutr 2007; 2: 125–126.
Giraudo SQ, Kotz CM, Grace MK, Levine AS, Billington CJ . Rat hypothalamic NPY mRNA and brown fat uncoupling protein mRNA after high-carbohydrate or high-fat diets. Am J Physiol 1994; 266: R1578–R1583.
Portillo MP, Serra F, Simon E, del Barrio AS, Palou A . Energy restriction with high-fat diet enriched with coconut oil gives higher UCP1 and lower white fat in rats. Int J Obes Relat Metab Disord 1998; 22: 974–979.
Roca P, Rodriguez AM, Oliver P, Bonet ML, Quevedo S, Pico C et al. Brown adipose tissue response to cafeteria diet-feeding involves induction of the UCP2 gene and is impaired in female rats as compared to males. Pflugers Arch 1999; 438: 628–634.
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26: 271–281.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011; 121: 96–105.
Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP et al. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes 2011; 60: 2465–2473.
Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J . Recruited vs. nonrecruited molecular signatures of brown, 'brite,' and white adipose tissues. Am J Physiol Endocrinol Metab 2012; 302: E19–E31.
Gummesson A, Jernas M, Svensson PA, Larsson I, Glad CA, Schele E et al. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. J Clin Endocrinol Metab 2007; 92: 4759–4765.
Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J et al. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. Embo J 2008; 27: 1537–1548.
Schwartz JH, Young JB, Landsberg L . Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Invest 1983; 72: 361–370.
Chida D, Hashimoto O, Kuwahara M, Sagara H, Osaka T, Tsubone H et al. Increased fat:carbohydrate oxidation ratio in Il1ra (−/−) mice on a high-fat diet is associated with increased sympathetic tone. Diabetologia 2008; 51: 1698–1706.
Watson E, Fargali S, Okamoto H, Sadahiro M, Gordon RE, Chakraborty T et al. Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol 2009; 9: 19.
Margareto J, Larrarte E, Marti A, Martinez JA . Up-regulation of a thermogenesis-related gene (UCP1) and down-regulation of PPARgamma and aP2 genes in adipose tissue: possible features of the antiobesity effects of a beta3-adrenergic agonist. Biochem Pharmacol 2001; 61: 1471–1478.
Moreno-Navarrete JM, Petrov P, Serrano M, Ortega F, Garcia-Ruiz E, Oliver P et al. Decreased RB1 mRNA, protein, and activity reflect obesity-induced altered adipogenic capacity in human adipose tissue. Diabetes 2013; 62: 1923–1931.
Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 2006; 55: 3229–3237.
Acknowledgements
CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of the ISCIII. This work was supported by the EU FP7 project DIABAT (HEALTH-F2-2011-278373) and by the Spanish Government (Ministerio de Educación y Ciencia, EPIMILK-AGL2012-33692). Laboratory of Molecular Biology, Nutrition and Biotechnology is member of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: FOOD-CT-2004-506360 NUGO). EGR and RDR are recipients of a fellowship from the University of the Balearic Islands and from the Spanish Government, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
García-Ruiz, E., Reynés, B., Díaz-Rúa, R. et al. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int J Obes 39, 1619–1629 (2015). https://doi.org/10.1038/ijo.2015.112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.112
This article is cited by
-
Anti-atherogenic and cardio-protective properties of sweet melon (Cucumis melo. L. Inodorus) seed extract on high fat diet induced obesity in male wistar rats
BMC Complementary Medicine and Therapies (2022)
-
Interaction between the amount of dietary protein and the environmental temperature on the expression of browning markers in adipose tissue of rats
Genes & Nutrition (2019)
-
High-fat feeding reprograms maternal energy metabolism and induces long-term postpartum obesity in mice
International Journal of Obesity (2019)
-
Effects of cold exposure revealed by global transcriptomic analysis in ferret peripheral blood mononuclear cells
Scientific Reports (2019)
-
Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-α cell signaling pathway
Endocrine (2019)