Abstract
The aim of this study was to determine the predictive value of the combination of serum angiopoietin-2 (Ang-2) levels and uterine artery Doppler for the detection of preeclampsia in women at 16–18 weeks of gestation and to identify other pregnancy complications that could be predicted with these combined tests. Maternal serum Ang-2 levels were measured, and uterine artery Doppler was performed in 400 pregnant women. The main outcome was preeclampsia. The predictive values of this combination were calculated. Twenty-five women (6.3%) developed preeclampsia. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of uterine artery Doppler combined with serum Ang-2 levels for the prediction of preeclampsia were 24.0%, 94.4%, 22.2% and 94.9%, respectively. For the prediction of early-onset preeclampsia, the sensitivity, specificity, PPV and NPV were 57.1%, 94.1%, 14.8% and 99.2%, respectively. Patients with abnormal uterine artery Doppler and abnormal serum Ang-2 levels (above 19.5 ng ml−1) were at higher risk for preterm delivery (relative risk=2.7, 95% confidence interval 1.2–5.8). Our findings revealed that the combination of uterine artery Doppler and serum Ang-2 levels at 16–18 weeks of gestation can be used to predict early-onset preeclampsia but not overall preeclampsia. Thus, this combination may be a useful early second trimester screening test for the prediction of early-onset preeclampsia.
Similar content being viewed by others
Introduction
Preeclampsia, a syndrome characterized by hypertension and proteinuria, affects approximately 5–10% of all pregnancies. It is a major obstetric complication and is the most common cause of fetal and maternal morbidity and mortality.1, 2 Early detection of preeclampsia and the identification of pregnant woman at high risk of developing preeclampsia may reduce maternal and neonatal morbidity and mortality.
Although the etiology of preeclampsia is still unknown, a two-stage model has been proposed,3 in which impairment of trophoblastic invasion in the spiral arteries causes them to have small lumina and high resistance. This model can be evaluated by uterine artery Doppler.4 Many uterine artery Doppler studies have shown that preeclamptic patients have abnormal uterine artery Doppler results more frequently than normal patients. Systematic reviews and meta-analyses have suggested that the presence of a uterine artery notch or increased uterine artery pulsatility index (PI) in the second trimester is better at predicting preeclampsia than in the first trimester (sensitivity 77–96%).5, 6 Previous studies in our population have shown that uterine artery PIs above the 95th percentile or bilateral diastolic notches had a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the prediction of preeclampsia of 20–75%, 52.5–95.8%, 9.1–14.3% and 97.1–97.2%, respectively.7, 8
Another model proposes angiogenic imbalance. The process of placental development, the coordination of vascular changes and the regulation of trophoblast growth are mediated by several locally acting angiogenic and anti-angiogenic factors and their receptors.9 When these processes are suboptimal, placental dysfunction can occur, causing preeclampsia. This can be evaluated by the measurement of angiogenic factors. In the maternal circulation of women with preeclampsia, Sahay et al.10 have found low levels of proangiogenic factors (vascular endothelial growth factor and placental growth factor) and high levels of angiotensin II type 1 receptor autoantibodies and anti-angiogenic factors (soluble fms-like tyrosine kinase-1 (sFlt-1) and the sFlt-1/placental growth factor ratio) during early gestation. One study has also demonstrated that enhanced oxidative stress in preeclamptic women during pregnancy may impair endothelial function, which then improves after delivery.11
Angiopoietin-2 (Ang-2) is produced by endothelial cells. It has been postulated that Ang-2 has a role in angiogenesis in abnormal pregnancy.12 Ang-2 promotes placental remodeling. Normal pregnancy is characterized by an increase in Ang-2 levels, with significantly lower levels found in pregnancies affected by placental dysfunction, leading to preeclampsia and intrauterine growth retardation.13, 14, 15 The predictive value of serum Ang-2 in preeclampsia is dependent on gestational age and the serum Ang-2 cutoff value. At 25–40 weeks of gestation, a serum Ang-2 cutoff value below 6.5 ng ml−1 showed an 82.8% sensitivity and 71.8% specificity for differentiating preeclamptic women from healthy pregnant women.14 In a previous study, maternal serum Ang-2 levels were similar between preeclamptic women and healthy pregnant women at 12–15 weeks of gestation; however, at 16–18 weeks of gestation, serum Ang-2 levels were higher in women who later developed preeclampsia using a cutoff level above 17.7 ng ml−1 with an odds ratio of 4.2 (95% confidence interval 1.4–12.6).16
Combinations of uterine artery Doppler with biochemical markers have higher predictive values for preeclampsia compared with single tests. Because of limitations related to the heterogeneity of the study population and the fact that most tests can better detect early-onset preeclampsia, the best predictive test for preeclampsia is still questionable.17, 18, 19 No studies have examined the combination of uterine artery Doppler and serum Ang-2 levels for the prediction of preeclampsia in pregnant women in the early second trimester.
The objective of this study was to determine the predictive value of the combination of serum Ang-2 levels and uterine artery Doppler for the detection of preeclampsia in women at 16–18 weeks of gestation and to identify other pregnancy complications that can be predicted with these combined tests.
Methods
This prospective observational study was performed at the Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand from January 2013 to December 2013. This study was approved by the Research Ethics Committee of the Faculty of Medicine, Chulalongkorn University. Written informed consent was obtained from all subjects.
Pregnant women with a gestational age of 16–18 weeks were invited to participate in this study. Women with fetal anomalies, medical diseases (chronic hypertension and renal disease) or a history of aspirin use, as well as women for whom we were unable to perform uterine artery Doppler, were excluded from this study. Gestational age was calculated from the last menstrual period and was confirmed by first trimester ultrasound.
Sample size calculation was based on the sensitivity for the prediction of preeclampsia reported by Hirokoshi et al.14 Accordingly, 394 women were required for this study.
Uterine artery Doppler evaluation
Uterine artery flow velocity waveforms were obtained by using ultrasonographic machines (GE Voluson 730 Pro or GE Voluson 730 Expert, GE Medical Systems, Zipf, Austria) with a convex abdominal probe AB 2–7 MHz. Each subject was examined once in the semi-recumbent position after 5 min of bed rest. The uterine artery was visualized by placing the end of the transducer on the left or right lower quadrant of the abdominal wall with the intent of identifying the external iliac artery and the uterine artery, which is medial to the external iliac artery. Flow velocity waveforms were obtained from each uterine artery where it crossed the external iliac artery. At least three waveforms from each side were recorded. Mean PI was calculated, and the presence or absence of an early diastolic notch was noted. A uterine notch was defined as a definite upward change in velocity after the deceleration slope of the primary wave. An abnormal uterine artery Doppler pattern was defined as a mean PI>95th percentile for each gestational age or the presence of bilateral early diastolic notches.18 One operator (PP) performed the measurements. The intra-operator coefficient of variation was calculated in 20 women, and each of these 20 women was examined three times. The mean intra-observer coefficient of variation for the PI was 0.96 (95% confidence interval=0.94–0.97).
Sample collection and Ang-2 immunoassay
After Doppler examination, venipuncture was performed, and blood was collected into clotted tubes. Blood samples were centrifuged at 2500 r.p.m. for 10 min and stored at −80 °C until assayed. Maternal serum Ang-2 levels were measured by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendations. The enzyme-linked immunosorbent assay kit is an enzymatically amplified two-step sandwich-type immunoassay. In this assay, the standards, controls and serum samples were incubated in microtitration wells coated with a monoclonal antibody against Ang-2. After incubation and washing, the wells were treated with Ang-2 conjugate. After a second incubation and washing step, the wells were incubated with the substrate solution. An acidic stopping solution was then added, and the degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurements at 450 and 540 nm using a microplate reader. The minimal detectable concentration in the assays for Ang-2, as reported by the manufacturer, was 0.012 ng ml−1. The inter-assay and intra-assay coefficients of variation were <10%.
Study outcomes
The primary outcome was the diagnosis of preeclampsia. Secondary outcomes included preterm delivery, intrauterine growth restriction, gestational diabetes, neonatal respiratory distress syndrome and perinatal death.
Statistical analysis
Data were analyzed with the SPSS software package version 17.0 for Windows (SPSS, Chicago, IL, USA) and are expressed as means, s.d.s, sensitivities, specificities, PPVs and NPVs, as well as relative risks with 95% confidence intervals. The optimal cutoff values for Ang-2 were calculated using the receiver operator characteristic curve. A chi-square test and Fisher’s exact test were used for categorical variables; the independent t-test was used for continuous variables, and the Mann–Whitney U test was used for nonparametric variables when appropriate. A P-value<0.05 was considered statistically significant.
Results
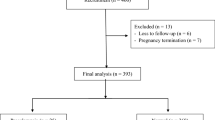
A total of 430 pregnant women were enrolled in this study, with 30 patients ultimately being excluded. Data from 400 patients were analyzed, with 25 women developing preeclampsia (6.3%), 16 developing mild preeclampsia (4%), 9 developing severe preeclampsia (2.3%) and 7 developing early-onset preeclampsia (1.7%). Regarding clinical characteristics, pregnant women with preeclampsia had a higher body mass index than normal pregnant women (Table 1). Pregnant women with preeclampsia had higher rates of preterm delivery, intrauterine growth restriction, neonatal respiratory distress syndrome, lower Apgar scores at the 1st minute after birth and lower birth weights than normal pregnant women (Table 2).
Pregnant women with preeclampsia had higher mean PIs of the uterine artery (1.30±0.45 vs. 1.05±0.39, P=0.003) and higher detection rates of unilateral (40.0 vs. 8.8%, P<0.001) and bilateral diastolic notching (28.0 vs. 4.0%, P<0.001) than normal pregnant women. Using uterine artery Doppler (PI above 95th percentile or bilateral diastolic notches) for the prediction of overall preeclampsia (Table 3), the sensitivity, specificity, PPV and NPV were 44%, 88.8%, 20.7% and 95.9%, respectively. For the prediction of early-onset preeclampsia, the sensitivity, specificity, PPV and NPV were 71.4%, 87.7%, 9.4% and 99.4%, respectively (Table 3).
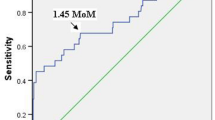
Pregnant women with overall preeclampsia or early-onset preeclampsia did not have significantly higher serum Ang-2 levels than pregnant women without preeclampsia (median 17.7 vs. 17.3 ng ml−1, P=0.79 and 22.5 vs. 17.7 ng ml−1, P=0.09, respectively). The optimal cutoff value of 19.5 ng ml−1 for serum Ang-2 level was based on the receiver operator characteristic curve. Using a serum Ang-2 cutoff value above 19.5 ng ml−1 for the prediction of overall preeclampsia, the sensitivity, specificity, PPV and NPV were 36%, 60.3%, 5.7% and 93.4%, respectively. For the prediction of early-onset preeclampsia, the sensitivity, specificity, PPV and NPV were 71.4%, 61.1%, 3.2% and 99.2%, respectively (Table 3).
Using uterine artery PIs above the 95th percentile or bilateral diastolic notches combined with serum Ang-2 levels with a cutoff value of 19.5 ng ml−1 for preeclampsia prediction, the sensitivity, specificity, PPV and NPV were 24%, 94.4%, 22.2% and 94.9%, respectively (Table 3). For early-onset preeclampsia prediction, the sensitivity, specificity, PPV and NPV were 57.1%, 94.1%, 14.8% and 99.2%, respectively (Table 3).
Patients with an abnormal uterine artery PI (PI above 95th percentile or bilateral diastolic notches) and an abnormal serum Ang-2 level (above 19.5 ng ml−1) were at a higher risk for preterm delivery (relative risk=2.7, 95% confidence interval 1.2–5.8) (Table 4).
Discussion
This study demonstrates that the combination of uterine artery Doppler and serum Ang-2 levels is not effective as a second trimester screening test for preeclampsia; however, this combination test is effective at predicting early-onset preeclampsia.
In this study, the combination of uterine artery Doppler and serum Ang-2 level was not effective as a second trimester screening test for preeclampsia. This may be because of the several complex mechanisms involved in the development of preeclampsia.20 Uterine artery Doppler and serum Ang-2 level may predict preeclampsia independently and in different manners. Thus, when these tests are used in combination to predict preeclampsia, they may not complement each other, resulting in reduced effectiveness. This finding was consistent with those of previous studies that have combined uterine artery Doppler with angiogenic factors and have found that the combination test did not improve the predictability of preeclampsia compared with single tests18, 21, 22 but contrasted with the findings of other studies.23, 24
The combination of uterine artery Doppler and serum Ang-2 levels at 16–18 weeks of gestation for the prediction of preeclampsia had a lower sensitivity and specificity compared with previous studies of the combination of uterine artery Doppler with other angiogenic factors.23, 24 This difference may be explained by differences in the gestational ages, differences in the study populations and differences in the angiogenic factors examined by our study and previous studies.
However, no studies of the combination of uterine artery Doppler and serum Ang-2 levels for the prediction of preeclampsia in pregnant women between 16 and 18 weeks of gestation have been conducted previously. This study found that the combination of uterine artery Doppler and serum Ang-2 level had good sensitivity and high specificity for the prediction of early-onset preeclampsia but not for the prediction of overall preeclampsia. This finding was similar to those of previous studies that have found that the combination of uterine artery Doppler and biochemical markers predicts early-onset preeclampsia better than overall preeclampsia.18, 25, 26, 27 Specifically, biochemical markers and uterine artery Doppler results were altered in cases of early-onset preeclampsia more than in cases of late onset preeclampsia.18, 25, 26, 28 A recent study has also demonstrated that the combination of pulse wave velocity with soluble fms-like tyrosine kinase-1 improves screening efficacy for predicting early-onset preeclampsia.29
In this study, serum Ang-2 levels at 16–18 weeks of gestation were higher in pregnant women with preeclampsia than in normal pregnant women. This finding was inconsistent with previous findings.16 The mechanism underlying the increased Ang-2 levels before the onset of preeclampsia remains unclear.16 As a result of abnormal placentation in early pregnancy, hypoxic conditions in the placentas of preeclamptic patients may activate Ang-2 expression.30 Ang-2 levels increase before the onset of preeclampsia. This finding is consistent with the increases in other angiogenic factors before the onset of preeclampsia.31, 32
Our unique study used a combination test of uterine artery Doppler and serum Ang-2 for the prediction of preeclampsia during a second trimester ultrasonographic examination for fetal anomaly screening. A notable limitation of this study was the time of measurement. We decided to conduct our measurements at 16–18 weeks gestation in conjunction with the ultrasonographic examination. This was more convenient for the patient because two screenings were performed in only one visit. It should be noted that if this combination test was performed at an earlier time, aspirin may have been prescribed before 16 weeks of gestation to prevent preeclampsia. Conversely, if these tests were performed later (such as late in the second trimester), they may have predicted preeclampsia more accurately.18 Further prospective study of this combination test at different time points during pregnancy should be conducted to evaluate the usefulness of these tests. Another limitation of this study was the small number of patients with early-onset preeclampsia. Further prospective study of this combination test should be conducted to confirm the usefulness of these tests for the prediction of early-onset preeclampsia.
The clinical application of this study is the identification of patients at risk for subsequent development of early-onset preeclampsia by using this combination test. This will be important for the timely referral of patients at high risk of developing early preeclampsia and for identification of the need for surveillance and starting antenatal corticosteroids at the appropriate time to induce fetal lung maturity. This approach will help to reduce maternal and neonatal morbidities in early-onset preeclampsia.
A recent publication has revealed that abnormal pressure-wave reflection from 26 to 32 weeks of gestation showed a stronger correlation with birth weight than conventional brachial blood pressure. This finding may provide new insight into the pathophysiology of fetal growth restriction, as well as superimposed preeclampsia, in pregnancies complicated by chronic hypertension.33
In conclusion, the combination of uterine artery Doppler and serum Ang-2 levels in pregnant women at 16–18 weeks of gestation can be used for the prediction of early-onset preeclampsia but not overall preeclampsia. This combination test may be useful early second trimester screening tests for the prediction of early-onset preeclampsia.
References
Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF . WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367: 1066–1074.
Bellamy L, Casas JP, Hingorani AD, Williams DJ . Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335: 974.
Roberts JM, Hubel CA . The two stage model of preeclampsia: variations on the theme. Placenta 2009; 30 (Suppl A): S32–S37.
Phupong V, Dejthevaporn T, Tanawattanacharoen S, Manotaya S, Tannirandorn Y, Charoenvidhya D . Predicting the risk of preeclampsia and small for gestational age infants by uterine artery Doppler in low-risk women. Arch Gynecol Obstet 2003; 268: 158–161.
Lovgren TR, Dugoff L, Galan HL . Uterine artery Doppler and prediction of preeclampsia. Clin Obstet Gynecol 2010; 53: 888–898.
Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, Khan KS . Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ 2008; 178: 701–711.
Phupong V, Dejthevaporn T . Predicting risks of preeclampsia and small for gestational age infant by uterine artery Doppler. Hypertens Pregnancy 2008; 27: 387–395.
Sritippayawan S, Phupong V . Risk assessment of preeclampsia in advanced maternal age by uterine arteries Doppler at 17-21 weeks of gestation. J Med Assoc Thai 2007; 90: 1281–1286.
Kaufmann P, Mayhew TM, Charnock-Jones DS . Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004; 25: 114–126.
Sahay AS, Patil VV, Sundrani DP, Joshi AA, Wagh GN, Gupte SA, Joshi SR . A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens Res 2014; 37: 753–758.
Watanabe K, Mori T, Iwasaki A, Kimura C, Matsushita H, Shinohara K, Wakatsuki A . Increased oxygen free radical production during pregnancy may impair vascular reactivity in preeclamptic women. Hypertens Res 2013; 36: 356–360.
Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB . Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab 2002; 87: 4213–4224.
Hirokoshi K, Maeshima Y, Kobayashi K, Matsuura E, Sugiyama H, Yamasaki Y, Masuyama H, Hiramatsu Y, Makino H . Increase of serum angiopoietin-2 during pregnancy is suppressed in women with preeclampsia. Am J Hypertens 2005; 18: 1181–1188.
Hirokoshi K, Maeshima Y, Kobayashi K, Matsuura E, Sugiyama H, Yamasaki Y, Masuyama H, Hiramatsu Y, Makino H . Elevated serum sFlt-1/Ang-2 ratio in women with preeclampsia. Nephron Clin Pract 2007; 106: c43–c50.
Wang Y, Tasevski V, Wallace EM, Gallery ED, Morris JM . Reduced maternal serum concentrations of angiopoietin-2 in the first trimester precede intrauterine growth restriction associated with placental insufficiency. Bjog 2007; 114: 1427–1431.
Leinonen E, Wathen KA, Alfthan H, Ylikorkala O, Andersson S, Stenman UH, Vuorela P . Maternal serum angiopoietin-1 and -2 and tie-2 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab 2010; 95: 126–133.
Tuuli MG, Odibo AO . The role of serum markers and uterine artery Doppler in identifying at-risk pregnancies. Clin Perinatol 2011; 38: 1–19 v.
Kulmala L, Phupong V . Combination of plasma-soluble fms-like tyrosine kinase 1 and uterine artery Doppler for the prediction of preeclampsia in cases of elderly gravida. Hypertens Res 2014; 37: 538–542.
Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC . Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv 2011; 66: 225–239.
Dekker GA, Sibai BM . Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 1998; 179: 1359–1375.
Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S . Combination of uterine artery Doppler velocimetry and maternal serum placental growth factor estimation in predicting occurrence of pre-eclampsia in early second trimester pregnancy: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol 2012; 161: 144–151.
Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G . Circulating angiogenic factors and abnormal uterine artery Doppler velocimetry in the second trimester. Hypertens Pregnancy 2006; 25: 183–192.
Diab AE, El-Behery MM, Ebrahiem MA, Shehata AE . Angiogenic factors for the prediction of pre-eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. Int J Gynaecol Obstet 2008; 102: 146–151.
Stepan H, Unversucht A, Wessel N, Faber R . Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007; 49: 818–824.
Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH . First-trimester prediction of hypertensive disorders in pregnancy. Hypertension 2009; 53: 812–818.
Raymond D, Peterson E . A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 2011; 66: 497–506.
Myatt L, Clifton R, Roberts J, Spong C, Wapner R, Thorp J Jr, Mercer B, Peaceman A, Ramin S, Carpenter M, Sciscione A, Tolosa J, Saade G, Sorokin Y, Anderson G . Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG 2013; 120: 1183–1191.
Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E . Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2008; 31: 303–309.
Katsipi I, Stylianou K, Petrakis I, Passam A, Vardaki E, Parthenakis F, Makrygiannakis A, Daphnis E, Kyriazis J . The use of pulse wave velocity in predicting pre-eclampsia in high-risk women. Hypertens Res 2014; 37: 733–740.
Han SY, Jun JK, Lee CH, Park JS, Syn HC . Angiopoietin-2: a promising indicator for the occurrence of severe preeclampsia. Hypertens Pregnancy 2012; 31: 189–199.
Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV . First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol 2008; 199: 266.e1–266.e6.
Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM . Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol 2008; 111: 1403–1409.
Tomimatsu T, Fujime M, Kanayama T, Mimura K, Koyama S, Kanagawa T, Endo M, Shimoya K, Kimura T . Abnormal pressure-wave reflection in pregnant women with chronic hypertension: association with maternal and fetal outcomes. Hypertens Res 2014; 37: 989–992.
Acknowledgements
This work was supported by a grant from the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (grant number RA57/054). We wish to thank the staff and nurses of the Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, for their helpful suggestions and assistance. We would also like to thank Mrs Rachanee Wongwathanavikrom, Ms Walailak Thongthab and Ms Natnicha Houngham for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Puttapitakpong, P., Phupong, V. Combination of serum angiopoietin-2 and uterine artery Doppler for prediction of preeclampsia. Hypertens Res 39, 95–99 (2016). https://doi.org/10.1038/hr.2015.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.113
Keywords
This article is cited by
-
Second-trimester serum high mobility group box-1 and uterine artery Doppler to predict preeclampsia
Scientific Reports (2022)
-
Serum SHARP1 and uterine artery Doppler for the prediction of preeclampsia
Scientific Reports (2019)
-
Novel biomarker profiles in experimental aged maternal mice with hypertensive disorders of pregnancy
Hypertension Research (2019)
-
Impairment of BKca channels in human placental chorionic plate arteries is potentially relevant to the development of preeclampsia
Hypertension Research (2018)
-
Combination of serum histidine-rich glycoprotein and uterine artery Doppler to predict preeclampsia
Hypertension Research (2018)