Abstract
Purpose
To compare the effect of intravitreal bevacizumab vsintravitreal triamcinolone for the treatment of non-ischaemic central retinal vein occlusion (CRVO).
Methods
The comparative nonrandomized retrospective clinical interventional study included 72 patients with non-ischaemic CRVO, divided into a bevacizumab group of 30 patients (1.25 mg bevacizumab) and a triamcinolone group of 42 patients (4.0 mg triamcinolone). All patients were consecutively included. At baseline, both study groups did not vary significantly in visual acuity, macular thickness, and duration of symptoms (191±300 days vs222±256 days). The minimal follow-up was 3 months (mean: 7.8±4.3 months; range: 3–12 months). During follow-up, 1.3±0.4 re-injections of the triamcinolone group (range:1–2 injections) and 2.7±1.8 re-injections of bevacizumab (range:1–6 injections) were administered.
Results
In both study groups, the mean visual acuity increased significantly (P<0.001) from baseline during follow-up. The differences in gain in visual acuity were not statistically significant (P>0.40) between both study groups at any time during follow-up. The mean macular thickness decreased significantly (P<0.001) in both study groups, with the reduction being significantly (P=0.006) more pronounced in the triamcinolone group. Intraocular pressure increased significantly (P<0.001) in the triamcinolone group.
Conclusions
In long-standing non-ischaemic CRVO, intravitreal bevacizumab and intravitreal triamcinolone are both associated with a comparable gain in visual acuity. The reduction in macular oedema was more marked in the triamcinolone group. In view of the potential complications of intravitreal triamcinolone in terms of intraocular pressure rise and cataractogenesis, bevacizumab may be preferred compared with triamcinolone for intravitreal use in non-ischaemic CRVO.
Similar content being viewed by others
Main
Retinal vein occlusions belong to the most common retinal disorders affecting the macula and reducing central visual acuity.1, 2, 3 In a recent population-based study, retinal vein occlusions were detected in about 0.7% of eyes of adult Chinese aged 40+ years. Branch retinal vein occlusions were about 12 times more common than central retinal vein occlusions (CRVOs), and the non-ischaemic type was about 9 times more common than the ischaemic type. From a pathogenic point of view, a decreased tissue perfusion and an increased hydrostatic pressure within the involved segments as a consequence of the vascular obstruction may lead to intraretinal haemorrhages, exudation of fluid, varying levels of tissue ischaemia, and eventually to intraocular neovascularization, if retinal ischaemia is pronounced.4
Although the Central Vein Occlusion Study Group has shown the beneficial of panretinal laser coagulation for the treatment of neovascularization, there have not been clear therapeutic recommendations for the treatment of macular oedema caused by CRVO.5 In the past 8 years, a change in a paradigm has taken place, now to consider the vitreous cavity as drug reservoir for the treatment of retinal disorders, such as diabetic retinopathy and retinal vein occlusions.6 The first drug, which was intravitreally injected was triamcinolone,7, 8, 9, 10, 11, 12, 13, 14 followed by ranibizumab and bevacizumab.15, 16, 17, 18, 19, 20, 21, 22 Both drugs differ in the spectrum of side effect and potentially in the magnitude and duration of their effect. As it has been unknown so far, which of the drugs may be preferable in which situation, we conducted a retrospective analysis comparing patients with non-ischaemic CRVO with respect to the change in visual acuity and intraocular pressure.
Materials and methods
The clinical interventional comparative retrospective non-randomized study included 72 eyes (72 patients) with non-ischaemic CRVO who consecutively underwent intravitreal injection of either triamcinolone (4.0 mg; 42 patients) or bevacizumab (1.25 mg; 30 patients) between May 2004 and October 2007, and who were followed for at least 3 months after the intravitreal injection. The diagnosis was substantiated by fluorescein angiography and optical coherence tomography showing significant cystoid macular oedema without marked retinal ischaemia, as defined by the Central Retinal Vein Occlusion Study Group.5 It was the decision of the attending retinologist whether triamcinolone or bevacizumab was injected, with the same retinologist using the same drug during the whole study period. The mean age was 55.64±16.26 years (mean±SD) in the triamcinolone group, and it was 54.67±15.50 years in the bevacizumab group without a significant difference between the two study groups (P=0.80) (Table 1). In a similar manner, both group did not vary significantly in the self-reported duration of the symptoms (P=0.51), and visual acuity (P=0.63), intraocular pressure (P=0.37), macular thickness (P=0.71) at baseline, and the number of patients with peripheral retinal laser coagulation therapies (P=0.62) (Table 1).
Inclusion criteria were significant macular oedema as measured by optical coherence tomography, loss of visual acuity, and macular vessel leakage in fluorescence angiography. Exclusion criteria were signs of any other fundus diseases (such as diabetic retinopathy); signs of non-perfusion or ischaemia, defined as neovascularization on the disc or elsewhere, iris neovascularization, or more than 10 disc areas of retinal non-perfusion detected by fluorescein angiography; and any earlier treatment (except of retinal laser coagulation) of the retinal vein occlusions, such as haemodiluting therapy or intravitreal injection of steroids or other antiangiogenic or antioedematous drugs. The study was approved by the local Institutional Review Board, and informed consent was obtained from every patient. The off-label use of bevacizumab and its potential risks and benefits were discussed in detail with the patients.
The technique of the intravitreal injections was similar as already reported in detail previously.7 The preservatives were removed. We applied a dosage of 4 mg of triamcinolone or of 1.25 mg of bevacizumab. At baseline, all patients underwent an ophthalmological examination including refractometry with assessment of best-corrected visual acuity, applanation tonometry, ophthalmoscopy, fluorescein angiography, and optical coherence tomography for measurement of the macular thickness.
After the intravitreal injection, the patients were scheduled to be re-examined at 1 day, 3 days, 1 month, 2, 3, 6 months, and 1 year after the injection. If postoperative problems or complications occurred, the follow-up examinations were carried out in shorter intervals. If during the follow-up the patients underwent ocular surgeries, only the results of the examination carried out earlier to that surgery were taken for the statistical analysis. The patients received re-injections when the macular oedema recurred. Recurrence of macular oedema was defined as a decrease in visual acuity associated with an increase of intraretinal or subretinal fluid as detected upon optical coherence tomography or fluorescein angiography. The interval between the first injection and repeated injections was at least 3 months for triamcinolone and it was at least 6 weeks for bevacizumab. There were 1.3±0.4 re-injection in the triamcinolone group (range: 1–2 injections), and 2.7±1.8 re-injection in the bevacizumab group (range: 1–6 injections).
Statistical analysis was carried out using a commercially available statistical software package (SPSS for Windows, version 16.0, SPSS, Chicago, IL, USA). Best-corrected visual acuity was converted into the logarithm of the minimum angle of resolution for statistical calculation.8 The data that were distributed normally were presented as the mean±SD. Where appropriate, the Student's t-test and the χ2-test were used. Confidence intervals were presented. All P-values were two-sided and were considered statistically significant when the values were less than 0.05.
Results
Both study groups did not vary significantly (P>0.15) in the preoperative data (Table 1). The mean follow-up was 7.8±4.3 months (range: 3–12 months).
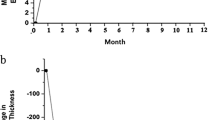
In both study groups, the mean visual acuity increased significantly (P<0.001) from baseline during the follow-up. In the triamcinolone group, the differences were significant for the comparisons between the baseline examination and the follow-up examination carried out at 2-months (P=0.03) and 3-months (P=0.02) after baseline (Figure 1). In the bevacizumab group, the differences were significant for the comparisons between the baseline examination and the follow-up examination carried out at 1 month (P=0.03), 6 months (P=0.04), and at 1 year (P=0.04) (Figure 1).
Comparing both study groups with each other, the differences in the gain of mean visual acuity were not statistically significant (P>0.40) at any time during follow-up examination (Table 2). In a similar manner, the percentage of patients who improved in best-corrected visual acuity by ⩾2 lines or who lost in best-corrected visual acuity ⩾2 lines was not significantly (P>0.30) different between both groups (Table 2). Correspondingly, the percentage of patients who improved in best-corrected visual acuity at 3 months follow-up by ⩾3 lines (19 (45%) patients in the triamcinolone group, 10 (33%) patients in the bevacizumab group; P=0.31) or who lost in best-corrected visual acuity at 3 months follow-up ⩾3 lines (2 (5%) patients in the triamcinolone group, 1 (3%) patients in the bevacizumab group; P=1.0) was not significantly different between both groups.
The mean macular thickness decreased significantly (P<0.001) in the triamcinolone study group from baseline to any re-examination carried out between 4 weeks and 1 year after the initial injection (Table 3). In the bevacizumab group, the reduction in central macular thickness was statistically significant for the follow-up examination carried out at 1 month, 2 months, and at 1-year follow-up (Table 3). The reduction in the macular thickness was significantly (P=0.006) more pronounced in the triamcinolone group than in the bevacizumab group at the 6 months follow-up examination (Figure 1).
In the triamcinolone group, two eyes (5%) developed iris neovascularization at 6 months after the initial injection, and two eyes (5%) developed a vitreous haemorrhage at 3 weeks and at 7 months after the initial injection, respectively. In the bevacizumab group, one eye (3%) developed iris neovascularization at 3 months after the initial injection and one eye (3%) developed a vitreous haemorrhage at 6 weeks after the initial injection
In the triamcinolone group, intraocular pressure readings higher than 21, 30, 35, and 40 mmHg, respectively, were measured in 18 eyes (43%), 6 eyes (14%), 4 eyes (10%), and 1 eye (2%), respectively, whereas in bevacizumab group, the intraocular pressure did not vary significantly between the examination at baseline and the examination during follow-up.
Discussion
Within the last 6 years, intravitreal triamcinolone has widely been used for the treatment of intraocular proliferative, oedematous, and neovascular diseases including CRVO.6, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 A disturbed balance of angiogenic and inflammatory cytokines has been reported to be associated with retinal vein occlusion,20 and experimental investigations and clinical studies have suggested a temporary antioedematous and antiangiogenic effect of intravitreal triamcinolone in eyes with CRVO.6, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The two major side effects of the intravitreal triamcinolone were a steroid induced increase in intraocular pressure and development of cataract.21, 22, 23, 24, 25 In contrast, studies on intravitreal bevacizumab by Rosenfeld et al and other researchers showed an improvement in visual acuity, reduction in macular thickness, and only minor complications in patients with CRVOs,26, 27, 28, 29, 30, 31, 32, 33, 34 so that intravitreal triamcinolone was rapidly exchanged by intravitreal bevacizumab for the treatment of CRVO. It agrees with this study, in which the best-corrected visual acuity improved significantly in the triamcinolone group and in the bevacizumab group with a no statistically significant difference in the gain in visual acuity between both study groups, although the reduction in macular oedema was slightly more pronounced in the triamcinolone group (Table 2) (Figure 1). The side effects in terms of an elevation in intraocular pressure were present in the triamcinolone group only, in a similar frequency and amount as already reported for Caucasians and in another study on Chinese patients.21, 22, 23, 24, 25
The finding of a discrepancy between a recurrence of macular oedema and continuously improved visual acuity agrees with an observation by Kriechbaum et al,32 in which 3 months after the injection macular oedema recurred and visual acuity remained unchanged.
The studies on the intravitreal use of bevacizumab for treatment of CRVO partially differ in the frequency of the bevacizumab application. In study by Kriechbaum et al,32 three initial injections were administered at 4-week intervals,32 although the intervals in other studies were usually 6 weeks to 2 months.34 In the investigation by Hsu et al,29 Iturralde et al,27 and in our study, only one initial injection was primarily given.
This study also agrees with a very recent investigation by Wu et al35 who compared intravitreal triamcinolone with intravitreal bevacizumab for treatment of macular oedema because of CRVO. The researchers concluded that intravitreal injection of triamcinolone or bevacizumab can both lead to a significant improvement in visual acuity and a resolution of macular oedema in patients with CRVO. However, the significant effect was not permanent. The efficacy of intravitreal triamcinolone acetonide showed no significant differences compared with intravitreal bevacizumab but seemed to cause more adverse events than bevacizumab.
There are limitations of our study. It is a hospital-based study so that without doubt a bias by the referral of patients was introduced. The self-reported duration of the symptoms was relatively long so that the results of our study may not be transferred for a fresh CRVO. Another weakness of our study was that some patients had undergone a retinal laser coagulation prior to be included into the study. The percentage of patients with a previous retinal laser coagulation was, however, not significantly different between both study groups (Table 1). Nonrandomization of the patients between the two study groups is another important limitation of our study. It was, however, the decision of the attending retinologist whether triamcinolone or bevacizumab was injected, with the same retinologist using the same drug during the whole study period. As the patients were randomly referred to the retinologists participating in the study, this flaw in the study design may not have markedly influenced the results of the investigation. Accordingly, the two study groups did not differ statistically significantly in their baseline parameters. Another limitation of the study is that intravitreal triamcinolone may have increased the formation or progression of cataract,22, 25 so that a vision-reducing effect of progressive of cataract might have hidden parts of a vision-improving effect of triamcinolone. Another limitation is the relatively long duration of symptoms before the treatment was carried out. The results of our study may, therefore, not directly be transferred to patients with a fresh onset of a CRVO. The relatively long duration of the symptoms before therapy was started may also be a reason why the macular thickness did not return to normal levels in all patients treated. Finally, one may consider that the patients were re-treated first when macular oedema returned. It differs from other treatment strategies in which three initial injections of bevacizumab are given in an interval of about 6–8 weeks. The treatment strategy in our study may, therefore, have led to an undertreatment.
In conclusion, in long-standing non-ischaemic CRVO, intravitreal bevacizumab and intravitreal triamcinolone are both associated with a comparable gain in visual acuity, although the reduction in macular oedema was more marked in the triamcinolone group. In view of the potential complications of intravitreal triamcinolone with respect to intraocular pressure elevation and cataract formation, bevacizumab may be preferred compared with triamcinolone for intravitreal use in non-ischaemic CRVO. If, however, intravitreal bevacizumab did not lead to an improvement in visual acuity, intravitreal triamcinolone may be tried, as a recent study suggested that in eyes with non-ischaemic CRVO in which intravitreal bevacizumab failed to improve vision intravitreal triamcinolone may lead to an increase in visual acuity.36
Summary

References
Mitchell P, Smith W, Chang A . Prevalence and associations of retinal vein occlusions in Australia. The blue mountains eye study. Arch Ophthalmol 1996; 114: 1243–1247.
Klein R, Klein BE, Moss SE, Meuer SM . The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Ophthalmologica 2000; 98: 113–143.
Liu W, Xu L, Jonas JB . Retinal vein occlusion Chinese subjects. Ophthalmology 2007; 114: 1795–1796.
Hayreh SS . Classification of central retinal vein occlusion. Ophthalmology 1983; 90: 458–474.
The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997; 115: 486–491.
Jonas JB . Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmol 2005; 83: 645–663.
Jonas JB, Kreissig I, Degenring RF . Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graef Arch Clin Exp Ophthalmol 2002; 240: 782–783.
Holladay JT . Visual acuity measurements. J Cataract Refract Surg 2004; 30: 287–290.
Greenberg PB, Martidis A, Rogers AH, Duker JS, Reichel E . Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol 2002; 86: 247–248.
Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol 2004; 122: 1131–1136.
Bashshur ZF, Ma’luf RN, Allam S, Jurdi FA, Haddad RS, Noureddin BN . Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol 2004; 122: 1137–1140.
Williamson TH, O’Donnell A . Intravitreal triamcinolone acetonide for cystoid macular edema in nonischemic central retinal vein occlusion. Am J Ophthalmol 2005; 139: 860–866.
Cekiç O, Chang S, Tseng JJ, Barile GR, Del Priore LV, Weissman H et al. Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina 2005; 25: 846–850.
Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF . Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. Eur J Ophthalmol 2005; 15: 751–758.
Avitabile T, Longo A, Reibaldi A . Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol 2005; 140: 695–702.
Ramezani A, Entezari M, Moradian S, Tabatabaei H, Kadkhodaei S . Intravitreal triamcinolone for acute central retinal vein occlusion; a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1601–1606.
Roth DB, Cukras C, Radhakrishnan R, Feuer WJ, Yarian DL, Green SN et al. Intravitreal triamcinolone acetonide injections in the treatment of retinal vein occlusions. Ophthalmic Surg Lasers Imaging 2008; 39: 446–454.
Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina 2006; 26: 896–901.
Gregori NZ, Rosenfeld PJ, Puliafito CA, Flynn Jr HW, Lee JE, Mavrofrides EC et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the management of macular edema secondary to central retinal vein occlusion. Retina 2006; 26: 889–895.
Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 2005; 140: 256–261.
Jonas JB, Kreissig I, Degenring R . Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 2003; 87: 24–27.
Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol 2004; 122: 336–340.
Smithen LM, Ober MD, Maranan L, Spaide RF . Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol 2004; 138: 740–743.
Lau LI, Chen KC, Lee FL, Chen SJ, Ko YC, Liu CJ, Hsu WM . Intraocular pressure elevation after intravitreal triamcinolone acetonide injection in a Chinese population. Am J Ophthalmol 2008; 146: 573–578.
Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM . Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 2005; 112: 139–143.
Rosenfeld PJ, Fung AE, Puliafito CA . Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 2005; 36: 336–339.
Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina 2006; 26: 279–284.
Spandau UH, Ihloff AK, Jonas JB . Intravitreal bevacizumab treatment of macular oedema due to central retinal vein occlusion. Acta Ophthalmol 2006; 84: 555–556.
Hsu J, Kaiser RS, Sivalingam A, Abraham P, Fineman MS, Samuel MA et al. Intravitreal bevacizumab (avastin) in central retinal vein occlusion. Retina 2007; 27: 1013–1019.
Priglinger SG, Wolf AH, Kreutzer TC, Kook D, Hofer A, Strauss RW et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: six-month results of a prospective trial. Retina 2007; 27: 1004–1012.
Pai SA, Shetty R, Vijayan PB, Venkatasubramaniam G, Yadav NK, Shetty BK et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol 2007; 143: 601–606.
Kriechbaum K, Michels S, Prager F, Georgopoulos M, Funk M, Geitzenauer W et al. Intravitreal avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol 2008; 92: 518–522.
Moschos MM, Moschos M . Intraocular bevacizumab for macular edema due to CRVO. A multifocal-ERG and OCT study. Doc Ophthalmol 2008; 116: 147–152.
Rensch F, Jonas JB, Spandau UH . Early intravitreal bevacizumab for non-ischemic central retinal vein occlusion. Acta Ophthalmol 2009; 87: 77–81.
Wu WC, Cheng KC, Wu HJ . Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema due to central retinal vein occlusion. Eye 2009. e-pub ahead of print 13 February 2009.
Jonas JB, Libondi T, Schlichtenbrede F, Schmidbauer M . Intravitreal triamcinolone after intravitreal bevacizumab for retinal vein occlusions. Acta Ophthalmol 2009. e-pub ahead of print 2 June 2009.
Acknowledgements
This study was supported by the Beijing Natural Science Foundation, Beijing, China (no: 7062065).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr J Jonas is a member of the advisory board for Posurdex (Allergen Inc., Irvine, CA, USA). The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tao, Y., Hou, J., Jiang, YR. et al. Intravitreal bevacizumab vs triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Eye 24, 810–815 (2010). https://doi.org/10.1038/eye.2009.220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.220
Keywords
This article is cited by
-
Results of intravitreal triamcinolone acetonide in patients with macular edema secondary to branch retinal vein occlusion
International Journal of Clinical Pharmacy (2014)
-
Visual prognostic value of photopic negative response and optical coherence tomography in central retinal vein occlusion after anti-VEGF treatment
Documenta Ophthalmologica (2013)
-
Editor's choice—top papers of 2010
Eye (2011)
-
Intravitreal bevacizumab for treatment of serous macular detachment in central retinal vein occlusion
Graefe's Archive for Clinical and Experimental Ophthalmology (2011)
-
Combined treatment of intravitreal bevacizumab and intravitreal triamcinolone in patients with retinal vein occlusion: 6 months of follow-up
Graefe's Archive for Clinical and Experimental Ophthalmology (2010)