Abstract
FAS/CD95/Apo-1 is a ubiquitously expressed cell-surface receptor involved in the initiation of programmed cell death. Its function in epidermal keratinocytes has been incompletely defined. Available evidence from in vitro studies points to important roles of Fas in the pathogenesis of contact dermatitis and in keratinocyte apoptosis induced by ultraviolet light. To define functions of Fas in the epidermis in vivo, we have generated mice with epidermis-specific deletion of the fas gene and tested its requirement for 2,4-dinitrofluorobenzene-induced contact dermatitis and for ultraviolet light B (UVB)-induced keratinocyte apoptosis. We report here our unexpected finding that keratinocyte apoptosis induced by both a contact allergen and UVB irradiation was significantly enhanced in Fas-negative epidermis. Expression of Fas by epidermal keratinocytes was neither necessary for the normal development of contact hypersensitivity of the skin, nor required for keratinocyte apoptosis following UVB irradiation. Our study results thus show that in the epidermis in vivo Fas exerts antiapoptotic effects that outweigh its proapoptotic role in contact hypersensitivity responses of the skin and in the tissue response of the epidermis to UVB irradiation.
Similar content being viewed by others
Main
Fas (APO-1/CD 95) is a widely expressed cell-surface receptor belonging to the tumor necrosis factor (TNF) receptor superfamily. The receptor chains are expressed on the cell surface as preassociated homotrimers. Binding of its cognate ligand, FasL, is thought to result in super clustering to form oligomeric receptor complexes. As a consequence, several proteins accumulate next to the cytoplasmic domain of Fas and form the death-inducing signaling complex (DISC).1 Two important components of the DISC are the adaptor protein Fas-associated death domain (FADD) and its binding partner caspase-8. Caspase-8 is a cysteine protease functioning as initiator of a cascade of proteolytic cleavage steps that finally leads to the activation of effector caspases. Effector caspases, such as the cystein protease caspase-3, cleave various intracellular proteins thereby inducing cellular changes that cause the phenomenon of apoptosis. In cells, caspase-3 is sequestered as a zymogen (p32 caspase-3). On activation of the proapoptotic effector cascade, this is converted into active caspase-3 by proteolytic cleavage. Activation of caspase-3 can be detected in tissues and cells by immunostaining using cleavage site-specific antibodies.2
Epidermal keratinocytes have been shown to express Fas,3 and ligation of Fas with a specific antibody can induce apoptosis of keratinocytes in culture after preincubation with γ-interferon. In addition, supernatant from activated human T lymphocytes containing both FasL and γ-interferon induced apoptosis of cultured human keratinocytes. Apoptosis of Fas-expressing epidermal keratinocytes in association with T-lymphocytes-expressing FasL has also been described in contact dermatitis and atopic dermatitis and was thought to be important in the pathogenesis of these conditions.4 The dissociation of epidermal keratinocytes with breakup of cell–cell contacts and formation of intra epidermal blisters, a phenomenon called spongiosis, has been thought to be mediated by Fas/FasL interactions. In addition to Fas/FasL interactions, perforin expressed by cytotoxic T lymphocytes can cause killing of keratinocytes. This additional mechanism of killing was shown to be of relevance in contact dermatitis in mice.5, 6
In addition to its role in initiating apoptosis, Fas signaling has also been proposed to activate non-cell autonomous antiapoptotic mechanisms in eczematous dermatitis. On induction of Fas signaling, epidermal keratinocytes produce ligands for the epidermal growth factor receptor (EGFR) such as amphiregulin, transforming growth factor-α (TGF-α) and others. These have been shown to activate antiapoptotic EGFR signaling in neighboring keratinocytes to protect them from apoptosis and thus restrict tissue damage. In addition, EGFR-dependent signals induced by Fas stimulation in keratinocytes have been implicated in the production of proinflammatory cytokines that could contribute to the inflammatory reaction in eczematous dermatitis.7, 8
Keratinocyte apoptosis in skin exposed to ultraviolet light (UV light) is characterized by the formation of sunburn cells (SBC). This is thought to be the main mechanism by which potentially transforming mutations are eliminated from the skin. UV light induces DNA damage that leads, in case of irreparability, to p53-mediated apoptosis of the damaged cell.9 The activity of DNA repair mechanisms, which determines whether keratinocytes undergo apoptosis, can be regulated by factors of the extracellular milieu.10 In addition to this DNA damage response, apoptosis of epidermal keratinocytes induced by UV light is regulated by Fas-dependent mechanisms.11 Irradiation of human epidermal keratinocytes with UVB induces clustering of Fas on the cell surface in a ligand-independent manner and recruitment of FADD to the cytoplasmic domain of Fas. This is thought to trigger the same caspase-8-dependent proapoptotic signaling cascade that is also induced after binding of FasL to Fas and leads to the activation of caspase-3.11, 12 Similar results have been described for murine epidermal keratinocytes.13, 14 In line with this proposed mechanism, studies in mice with mutated FasL suggested that upregulation of Fas and FasL expression after irradiation with high doses of UVB contribute to apoptosis of epidermal keratinocytes.15 However, UVB-induced keratinocyte apoptosis has also been described to proceed through the internal, mitochondrial pathway. UV light is thought to damage the mitochondrial membrane thus leading to the activation of caspase-9, a critical step in the initiation of apoptosis via this pathway. Blocking caspase-9 as well as targeted deletion of Jun N-terminal kinase can inhibit UV-induced apoptosis,16, 17 whereas blocking of caspase-8 did not prevent UV-dependent apoptosis in murine embryonic fibroblasts (MEFs).18, 19 This suggested that caspase-8-mediated Fas signaling may not be essential for the proapoptotic response to UVB irradiation in MEFs.
Given partially contradictory results regarding functions of Fas in keratinocytes, we decided to use conditional gene targeting technology to further elucidate the role of Fas in the epidermis in vivo.
Results
Generation of epidermis-specific Fas knockout mice
Mice with conditional ablation of Fas alleles have been described previously.20 Female mice homozygous or heterozygous for the floxed (Fl) Fas allele were bred to male mice homozygous or heterozygous for the Fl Fas allele and expressing Cre recombinase under the control of the keratin 14 promoter.21 Samples of epidermis from ear and back skin as well as total tissue lysates from mouse tails for comparison were analyzed for the presence of wild-type and mutant Fas DNA (Supplementary Figure 1a) and of Fas protein using PCR and western blotting, respectively. Whereas epidermal samples from ear and back skin showed complete deletion of Fl Fas alleles, Fas was readily detected in total tissue lysates from tails (Figure 1a; Supplementary Figure 1b). This showed epidermis-specific deletion of Fas in mice with the genotype K14Cre+ Fasfl/fl, which will be referred to as FasE-KO mice. Mice with the genotypes K14Cre− Fasfl/fl, K14Cre− Fasfl/+, K14Cre+ Fas+/+ and K14Cre− Fas+/+ are referred to as control mice. Mice with the genotype K14Cre+ Fasfl/+ were excluded from the analysis.
Deletion of Fas in epidermal keratinocytes. Western blot (a, d) and FACS analyses (b) with antibodies against Fas (upper panels) and actin as loading control (lower panels). (a) Epidermis isolated from back skin as well as whole tissue of tails was used. (b, d) Primary epidermal keratinocytes isolated from newborn mice were analyzed for Fas expression by FACS analysis (b) and western blot (d). (c) Primary control and Fas KO keratinocytes were treated with 10 ng/ml IFN-γ for 48 h and then stimulated with 50 ng/ml of the agonistic Fas antibody Jo2 or left unstimulated. After 8 and 24 h, cultures were analyzed for TUNEL-positive cells. Bar graphs show percent increase in the number of TUNEL-positive cells after Jo2 stimulation in comparison to unstimulated cells. ct, control mice/keratinocytes; Thy, thymus extract as positive control; unst, unstained; sec AB, secondary antibody
We also analyzed primary keratinocytes isolated from control and FasE-KO mice. Western blot and FACS analyses showed expression of Fas by keratinocytes from control mice. This was not detectable in keratinocytes isolated from FasE-KO mice (Figure 1b and d). In addition, we tested the response of interferon-γ-treated control and Fas knockout (KO) keratinocytes to stimulation of Fas using the agonistic antibody Jo2.22 Whereas control keratinocytes showed an increase in the percentage of apoptotic cells upon Jo2 stimulation, the number of apoptotic cells was not increased in the Fas KO keratinocyte population (Figure 1c).
FasE-KO mice did not show an overt phenotype (Supplementary Figure 2). Their skin was macroscopically and histologically indistinguishable from that of control mice. We also did not observe differences in the hair coat. Staining for markers of proliferation and differentiation in the skin of FasE-KO mice and control mice did not show any differences (Supplementary Figure 3).
Enhanced contact-dermatitis-induced keratinocyte apoptosis in FasE-KO mice
Fas has been proposed to exert an important effect on the pathogenesis of contact dermatitis by mediating apoptosis of epidermal keratinocytes.4 We induced a contact hypersensitivity (CH) reaction in the ears of 16 FasE-KO mice (9 females and 7 males) and 14 control mice (6 females and 8 males) by challenging them with 2,4-dinitrofluorobenzene (DNFB) in acetone/olive oil (4 : 1), 6 days after sensitization. Ear swelling was determined at different time points after challenge as a marker for the strength of the inflammatory reaction. Ear thickness was determined on the day of sensitization and immediately before challenge. The average of these measurements formed the baseline value for the determination of ear swelling. Measurements of ear thickness were repeated 24, 48, 72 and 96 h after challenge. No significant differences were detected between FasE-KO mice and control mice at any time point (Figure 2a). Females showed stronger ear swelling than males through all time points. Both FasE-KO mice and control mice showed edema and an inflammatory infiltrate in the dermis with similar density and comparable numbers of inflammatory cells staining positive for CD3 (T lymphocytes) or CD11b (myeloid inflammatory cells) (Figure 2b). Similar numbers of F4/80-positive inflammatory cells (macrophages) were detected in the epidermis at sites of spongiosis both in control and FasE-KO mice.
DNFB induced contact dermatitis in the absence of epidermal Fas expression. (a) 16 FasE-KO mice (9 females and 7 males) and 14 control mice (6 females and 8 males) were challenged with DNFB 6 days after sensitization. Ear swelling was determined before (0 h) and 24, 48, 72 and 96 h after the challenge. Individual data points show the mean increase of ear swelling for each time point calculated from three consecutive measurements of ear thickness. (b) Micrographs and immunofluorescent images of ear skin of control and FasE-KO mice 96 h after challenge with DNFB. The lower panel of pictures of H/E- stained sections shows spongiotic blister formation. The immunostainings show green fluorescence for CD3 and CD11b. Red staining shows nuclei. Scale bars in H/E staining: upper, 100 μm; lower, 50 μm. Scale bar in immunofluorescent staining=100 μm
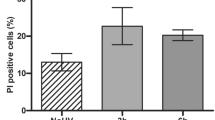
Spongiosis was present in all skin samples of challenged FasE-KO mice, to a similar extent as in control mice (Figure 2b). We used immunostaining of activated caspase-3 and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining (Figure 3a) to visualize intraepidermal apoptotic cells in challenged ears of FasE-KO mice and control mice 96 h after challenge. Unexpectedly, the number of apoptotic cells in the epidermis was higher in both female and male FasE-KO mice as compared with controls. The differences between FasE-KO mice and control mice were significant (Figure 3b). We conclude that in the DNFB-induced CH reaction in mice (1) Fas expressed by epidermal keratinocytes is dispensable for the ear swelling reaction, the accumulation of an inflammatory infiltrate and spongiosis, and (2) the presence of Fas in the epidermis partially protects epidermal keratinocytes from apoptosis.
Contact hypersensitivity induced keratinocyte apoptosis in Fas-negative epidermis. Apoptotic cells were detected 96 h after challenge by staining with an antibody against cleaved caspase-3 and by TUNEL staining. (a) Microscopic images of skin sections of FasE-KO mice and control mice stained for active caspase-3. Arrows indicate apoptotic cells within the epidermis. (b) Bar graphs show mean numbers±S.D. of caspase-3- or TUNEL-positive epidermal cells per power field in skin sections of male and female control and FasE-KO mice. A total of 160 power fields of caspase-3 and TUNEL-stained sections were counted. Asterisk (*) indicates statistical significance of the difference, P-values are given below. (The color reproduction of the Figure is available on the html full text version of the paper.)
Enhanced UVB-induced keratinocyte apoptosis in FasE-KO mice
Fas has also been implicated in the apoptosis of keratinocytes following UVB irradiation. We therefore irradiated 15 FasE-KO mice and 13 age-matched control mice with a dose of 1 J/cm2 UVB on their back after shaving. After 18 and 24 h, back skin from eight male FasE-KO mice and nine male control mice or seven female FasE-KO mice and four female controls was taken for analysis. Histological sections of paraffin-embedded skin were used for counting SBC and for TUNEL staining. Although there was no difference in the number of TUNEL-positive cells in the epidermis of control and FasE-KO mice, there were significantly more SBC present in the skin of FasE-KO mice (Figure 4b). Staining with an antibody against the macrophage marker F4/80 did not show positive cells within the epidermis. We conclude that expression of Fas by epidermal keratinocytes is not required for UVB-induced keratinocyte apoptosis in vivo and that the presence of Fas reduces the number of SBC upon UVB irradiation.
Apoptosis of epidermal keratinocytes on UVB irradiation in Fas-negative epidermis. Detection of SBC and TUNEL-positive keratinocytes 18 and 24 h after UVB irradiation. (a) High-power images of skin sections of FasE-KO mice and control mice 18 h after UVB irradiation-stained H/E (light microscopy) or with the TUNEL method (fluorescence microscopy). Black arrows indicate SBC, white arrows TUNEL-positive cells. (b) Bar graphs show mean numbers±S.D. of SBC per high-power field (upper panel) and TUNEL-positive epidermal cells per power field (lower panel) 18 and 24 h after UVB irradiation. To analyze numbers of SBC, we counted 170 and 110 power fields in the 18 and 24 h experiment, respectively. For TUNEL stainings, 196 (ct) and 252 (FasE-KO) power fields were counted. Asterisks (**) indicate statistical significance, P-value is given above. Differences in SBC number were not significant after 24 h. SBC, sunburn cells; HPF, high-power field; PF, power field
No requirement for Fas in UVB-induced keratinocyte apoptosis in vitro
In vivo, there is constant exchange of information between the epidermis and other skin compartments. To investigate whether deletion of Fas had an influence on cell autonomous mechanisms of keratinocyte apoptosis, we isolated keratinocytes from Fas-negative epidermis and control epidermis. Western blot and FACS analyses showed that keratinocytes isolated from FasE-KO mice (FasKO keratinocytes) did not express Fas protein (Figure 1b and d). Control and FasKO keratinocytes, three lines each, were then irradiated in vitro with 100 and 300 mJ/cm2 UVB and analyzed by FACS using an antibody against Annexin V and by TUNEL staining. Results of both Annexin V and TUNEL staining showed similar numbers of apoptotic control and FasKO keratinocytes (Figure 5). We conclude that apoptosis of keratinocytes on UVB irradiation does not depend on the presence of Fas.
UVB-induced apoptosis of Fas-negative keratinocytes in vitro. Primary murine keratinocytes in passages 2–4 isolated from control mice (ct) and FasE-KO mice (Fas KO) were irradiated with 100 or 300 mJ/cm2 UVB, respectively. Upper part: FACS analysis of Annexin V exposure on the cell surface was carried out after 16 h. Bar graphs show the percentage of Annexin-V-positive keratinocytes in the propidium-iodide-negative population±S.D. Results are from three control and Fas KO primary isolates analyzed in at least two independent experiments. Lower part: Primary keratinocytes were irradiated with 300 mJ/cm2 UVB and labeled with the TUNEL technique 16 h later. Bar graphs show the percentage of TUNEL-positive keratinocytes in the total adherent cell population±S.D. in two independent experiments using keratinocytes from three control and three FasE-KO mice
Discussion
Epidermal keratinocytes constitutively express CD953 and Fas-induced keratinocyte death is currently thought to have a relevant role in the pathogenesis of a number of severe skin diseases, such as eczema and toxic epidermal necrolysis.4, 23 Although apoptosis of epidermal keratinocytes is considered as a hallmark of eczematous dermatitis,24 the biological meaning of this is not well understood. The results from experimental models of contact dermatitis are inconclusive: the absence of Fas or FasL in mice has been reported to have no influence on the contact hypersensitivity response to DNFB.5 In another study, however, oxazolone-induced contact dermatitis was found to be attenuated in Fas mutant lpr mice.25 Because both studies were performed in animals with constitutive functional Fas or FasL deficiency, they were not able to specifically address contributions of epidermal keratinocytes to the tissue reaction. Our approach of an epidermis-specific deletion of Fas clearly shows that Fas expression by epidermal keratinocytes is dispensable for a normal contact hypersensitivity response, as ear swelling and inflammatory features were identical in control and Fas-deficient skin. The formation of spongiotic vesicles, which is seen as a characteristic sign of acute eczematous dermatitis and has been proposed to be induced by Fas-mediated keratinocyte killing,4 occurred normally in the absence of epidermal Fas expression, showing that it is not Fas dependent. It is likely that, in the absence of Fas, keratinocyte killing is executed by perforin/granzyme-dependent mechanisms.5
In addition to its role in the induction of apoptosis, Fas has been suggested to elicit antiapoptotic and proinflammatory signals in the epidermis through the production of EGF receptor ligands, such as amphiregulin, TGF-α and numerous proinflammatory cytokines.7, 8, 26 Recent work shows that the intracellular domain of Fas can be tyrosine phosphorylated, which can lead to the recruitment and activation of phosphatidylinositol-3-kinase (PI-3K).27 These findings are based on in vitro studies of immortalized and primary human keratinocytes and other cell types; their relevance for the in vivo situation within the epidermis remained therefore unclear. Our unexpected study result showing that keratinocyte apoptosis on DNFB challenge is enhanced in Fas-negative epidermis shows an antiapoptotic function of Fas and it is possible that PI-3K signaling or EGF receptor ligands mediate this protective signal. This mechanism could indeed restrict extensive tissue damage in response to proapoptotic stimuli, thus contributing to the maintenance of an intact skin barrier.7 Probably, this function of Fas is mediated in a non-cell autonomous juxtacrine way, as we did not detect the protective effect against apoptosis in cultures of FasKO keratinocytes. Although this is, in our view, the most likely mechanism explaining the accumulation of apoptotic cells in the epidermis of FasE-KO mice, we cannot completely exclude the possibility that the deficiency of Fas in epidermal keratinocytes leads to an inhibition of their autophagocytic activity. This would result in a decreased elimination of apoptotic cells and thereby in their accumulation. Such a function for Fas has, however, so far not been reported. In contrast, the death effector domain containing cellular FLICE-like inhibitor protein c FLIP, which can be recruited to the DISC through the adapter protein FADD upon Fas ligation, has been shown to suppress autophagy.28
Stimulation of Fas in epidermal keratinocytes has also been reported to result in the production of proinflammatory cytokines that could facilitate the inflammatory response in eczematous dermatitis.8, 26 On the basis of these findings, a possible pathogenic role of Fas-induced cytokine production in eczema has been assumed.29 We show here that epidermis-specific deficiency of Fas does not alter the contact hypersensitivity response to a DNFB challenge in mice. Although our study results do not exclude production of proinflammatory cytokines in the epidermis on Fas ligation, they show that the presence of Fas is not required for the pathogenesis of contact dermatitis in vivo.
Keratinocyte apoptosis on UV irradiation and subsequent elimination of apoptotic bodies from the skin are believed to be the mechanisms that protect the epidermis from cumulating DNA damage in sun-exposed skin. UV light is absorbed by nuclear, cytoplasmic or cell membrane-attached molecules that serve as chromophores. Excitation of these chromophores can activate a number of signaling pathways leading to apoptosis. Fas has been shown to cluster on the surface of human epidermal keratinocytes upon UVB irradiation in a FasL-independent manner. This is followed by the recruitment of the adapter molecule FADD to the receptor complex, which then enables the recruitment of caspase-8. Both a dominant-negative FADD construct and inhibition of caspase-8 could reduce UVB-induced apoptosis, suggesting a causal role of the Fas- FADD-caspase-8 interaction in the induction of apoptosis.11, 12, 30 This role of Fas was further supported by the finding that 500 mJ/cm2 UVB irradiation of C3H/HeJ mice with mutated FasL (gld/gld mice) resulted in less apoptosis as compared with wild-type controls.15 In contrast to these findings, we show here that in C57/Bl6 mice the Fas/FasL signaling system is not essentially involved in UVB-induced keratinocyte apoptosis. Both counting of SBC and TUNEL staining revealed that there is no decrease in the numbers of apoptotic keratinocytes in Fas-negative epidermis as compared to control epidermis. This is further supported by our in vitro analysis of UVB-irradiated Fas KO and control keratinocytes, which did not show differences in Annexin V and TUNEL staining. These results are consistent with data showing that deletion or blockade of caspase-8 does not prevent UVB-induced apoptosis of MEFs.18, 19 Furthermore, the increased number of SBC in the epidermis of FasE−KO mice suggests that, in vivo, Fas exerts antiapoptotic effects. TUNEL staining did not show a difference in the numbers of apoptotic keratinocytes between FasE-KO and control mice. This is most likely due to the relatively late time point of 18 h after irradiation, which was chosen primarily for the detection of SBC.
The difference between our study results and that from other models of UVB-induced keratinocyte apoptosis may be explained by variations in the UV response between mouse strains. Another explanation could be a gradual involvement of different proapoptotic mechanisms depending on the UVB dose, as Hill et al.15 used 500 mJ/cm2 whereas we used 300 mJ/cm2. A third hypothetical explanation is offered by the fact that gld/gld mice are not deficient for FasL but carry a point mutation within the protein that disables proapoptotic Fas signaling.31 As a consequence, mutant FasL mRNA is constitutively and strongly expressed in lymphoid cells of gld/gld mice.32 It is therefore conceivable that mutant FasL in these mice, although not able to stimulate proapoptotic Fas signaling, can still bind to CD95 expressed on the surface of epidermal keratinocytes and activate its antiapoptotic signaling mechanisms. This would then lead to the stimulation of survival signals in keratinocytes and thus result in decreased apoptosis. The ability of Fas to stimulate pathways involved in cell survival has been demonstrated recently.33 In addition, signaling through c-FLIP, which is known to exert strong antiapoptotic effects by binding to the adapter protein FADD and competing for the recruitment of caspase-8, could be a mechanism mediating antiapoptotic activities of Fas.34
In summary, we here provide genetic evidence showing conclusively that, in murine skin, Fas is an essential element of pathways that restrict keratinocyte apoptosis in eczematous dermatitis and on UVB irradiation. Neither contact allergen- nor UVB-induced keratinocyte apoptosis depend on the expression of Fas by epidermal keratinocytes in vivo. Moreover, both the inflammatory tissue reaction and spongiosis in acute eczematous dermatitis can proceed in the absence of epidermal Fas expression. The exact mechanisms of antiapoptotic Fas signaling remain unclear here; both Fas-mediated autocrine stimulation of EGF-receptor-dependent signals7 and activation of PI-3K33 could be relevant in this context. Both in contact dermatitis and on UVB irradiation the protective effects of Fas apparently outweigh Fas-induced keratinocyte death. It therefore seems possible that, for the maintenance of tissue integrity within the epidermis, antiapoptotic functions of Fas are at least as important as its proapoptotic functions.
Materials and Methods
Generation of mice with epidermis-specific Fas deficiency and UV irradiation
Mice with conditionally targeted Fas alleles have been described previously.20 These mice were crossed with mice expressing Cre recombinase under the control of the keratin 14 promoter.21 Deletion of Fl Fas alleles and presence of the Cre recombinase transgene were analyzed by PCR as described therein. For UVB treatment mice were anesthetized, shaved on their backs and irradiated with a dose of 300 mJ/cm2 UVB using a Waldmann UV 801 lamp (Waldmann, Villingen-Schwenningen, Germany) equipped with UV21 bulbs emitting UV light in the range of 280–360 nm with an emission maximum at about 314 nm. The UV dose was determined using a Variocontrol UV radiometer (Waldmann). Mice were killed after 18 or 24 h and skin was taken for analysis.
Western blot analysis of epidermis and cultured keratinocytes
Epidermis was separated from dermis with forceps after 30 min of incubation in 3.8% ammonium thiocyanate dissolved in borate buffer (pH 7.6) at 4°C. Epidermis samples were minced and homogenized for 2 min on ice using an MM300 ultrasound tissue homogenizer (Retsch GmbH, Haan, Germany), extracted for 1 h in modified RIPA buffer containing 5 mM EDTA, 1% Triton 100 × , 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 20 μM leupeptin, 1 mM PMSF, 0.5 mg/ml soybean trypsin inhibitor, 0.5 mM NaVO3 and 10 mg/ml p-nitrophenylphosphate, and centrifuged at 14 000 × g for 10 min at 4°C. The supernatant was used for protein analysis. Keratinocytes were lysed in situ using the same buffer, scraped from the dishes and sonicated for 30 s at full power. Lysates were centrifuged at 14 000 × g for 10 min and the supernatant was used for western blot analysis. Protein (30 μg) was separated by SDS-PAGE and blotted onto nitrocellulose LC 2000 membranes (Invitrogen, Carlsbad, CA, USA). After blocking with 5% milk powder solution the membrane was probed with the following antibodies: rabbit polyclonal anti-Fas M-20 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal anti-actin antibody (MP Biomedicals, Aurora, OH, USA). Immunoreactive proteins were visualized with HRP-coupled secondary antibodies on Hyperfilm by chemiluminescence (Western Lightning; PerkinElmer, Waltham, MA, USA). Protein quantification was carried out using the Pierce protein quantification kit (Pierce, Thermo Fisher Scientific, Rockford, IL, USA).
Induction of contact dermatitis
Mice were anesthetized on day 0 and the thickness of the left ear was measured twice. Mice were shaved on the abdomen and 25 μl of a 0.5% DNFB solution in acetone/olive oil (4 : 1) was applied and allowed to dry. After 6 days, the mice were again anesthetized and the thickness of the left ear was determined twice. The mean of thickness measurements on days 0 and 6 formed the 0 value for the stimulation experiment. A 0.2% DNFB solution (10 μl) was applied to the front and back sides of the left ear. On days 7–10 after sensitization, mice were anesthetized and the thickness of the left ear was determined twice. The relative increase in ear thickness was calculated for each time point.
Histopathological analysis and immunostaining
After excision, tissue samples were fixed in 4% paraformaldehyde or embedded in OCT compound and frozen immediately. Further processing, paraffin embedding of paraformaldehyde-fixed tissue and hematoxylin and eosin (H/E) staining, was carried out according to standard histopathological procedures. Immunostaining of keratin 14, keratin 10 and loricrin was performed as described in Stratis et al.35 For immunostaining against activated caspase-3 on paraffin-embedded tissue, a rabbit antibody against cleaved caspase-3 (Cell Signaling Technology Inc., Denver, CO, USA) at a dilution of 1 : 50 was used according to the manufacturer's instructions.
TUNEL staining
TUNEL staining of tissue sections and cells was carried out with the Dead End Fluorimetric TUNEL System (Promega Corporation, Madison, WI, USA) according to the manufacturer's instructions.
Isolation and culture of epidermal keratinocytes, UV irradiation and Fas stimulation
Epidermal keratinocytes were isolated from transgenic and wild-type newborn mice as described in Tscharntke et al.36 For UVB irradiation, keratinocytes were grown to subconfluency without feeder cells, washed with PBS and then irradiated with 100 or 300 mJ/cm2 UVB using a Bio Sun++ System at 312 nm (Vilber Lourmat, Marne-la-Vallée, France). After 16 h of culture keratinocytes were trypsinized and analyzed by FACS or TUNEL staining. For stimulation of Fas, primary epidermal keratinocytes were incubated with 10 ng/ml IFN-γ (kindly provided by Jonathan Howard, University of Cologne) to increase Fas expression. After 48 h cells were stimulated with 50 ng/ml Jo2 antibody (Becton Dickinson, Franklin Lakes, NJ, USA, catalog no. 554256) or left unstimulated. After 8 or 20 h keratinocytes were fixed and numbers of TUNEL-positive cells were determined.
FACS analysis
Keratinocytes were trypsinized and resuspended in HEPES incubation buffer. Staining of Annexin V was performed using the Annexin V FLUOS Staining Kit (Roche Applied Science, Penzberg, Germany). Analysis was performed with a FACSCalibur (Becton Dickinson).
Statistics
Determination of significance between samples was performed using the Student's t-test (GraphPad Prism software, GraphPad Software Inc., La Jolla, CA, USA). For all statistical tests, the 0.05 level of the confidence interval was accepted as statistically significant.
Abbreviations
- CH:
-
contact hypersensitivity
- DISC:
-
death-inducing signaling complex
- DNFB:
-
2,4-dinitrofluorobenzene
- EGFR:
-
epidermal growth factor receptor
- FADD:
-
Fas-associated death domain
- FasL:
-
Fas ligand
- Fl:
-
floxed
- H/E:
-
hematoxylin and eosin
- KO:
-
knockout
- MEFs:
-
murine embryonic fibroblasts
- PI-3K:
-
phosphatidylinositol-3-kinase
- SBC:
-
sunburn cells
- TGF-α:
-
transforming growth factor-α
- TNF:
-
tumor necrosis factor
- TUNEL:
-
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- UVB:
-
ultraviolet light B
References
Peter ME, Krammer PH . The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 2003; 10: 26–35.
Gown AM, Willingham MC . Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem 2002; 50: 449–454.
Matsue H, Kobayashi H, Hosokawa T, Akitaya T, Ohkawara A . Keratinocytes constitutively express the Fas antigen that mediates apoptosis in IFN gamma-treated cultured keratinocytes. Arch Dermatol Res 1995; 287: 315–320.
Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000; 106: 25–35.
Kehren J, Desvignes C, Krasteva M, Ducluzeau MT, Assossou O, Horand F et al. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med 1999; 189: 779–786.
Kagi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994; 265: 528–530.
Iordanov MS, Sundholm AJ, Simpson EL, Hanifin JM, Ryabinina OP, Choi RJ et al. Cell death-induced activation of epidermal growth factor receptor in keratinocytes: implications for restricting epidermal damage in dermatitis. J Invest Dermatol 2005; 125: 134–142.
Farley SM, Purdy DE, Ryabinina OP, Schneider P, Magun BE, Iordanov MS . Fas ligand-induced proinflammatory transcriptional responses in reconstructed human epidermis. Recruitment of the epidermal growth factor receptor and activation of MAP kinases. J Biol Chem 2008; 283: 919–928.
Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J et al. Sunburn and p53 in the onset of skin cancer. Nature 1994; 372: 773–776.
Schwarz A, Ständer S, Berneburg M, Böhm M, Kulms D, van Steeg H et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol 2002; 4: 26–31.
Kulms D, Pöppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T . Nuclear and cell membrane effects contribute independently to the induction of apoptosis in human cells exposed to UVB radiation. Proc Natl Acad Sci USA 1999; 96: 7974–7979.
Aragane Y, Kulms D, Metze D, Wilkes G, Pöppelmann B, Luger TA et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol 1998; 140: 171–182.
Takahashi H, Ishida-Yamamoto A, Iizuka H . Ultraviolet B irradiation induces apoptosis of keratinocytes by direct activation of Fas antigen. J Investig Dermatol Symp Proc 2001; 6: 64–68.
Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H . Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res 1999; 249: 291–298.
Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB . Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science 1999; 285: 898–900.
Sitailo LA, Tibudan SS, Denning MF . Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem 2002; 277: 19346–19352.
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 2000; 288: 870–874.
Naik E, Michalak EM, Villunger A, Adams JM, Strasser A . Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol 2007; 176: 415–424.
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9: 267–276.
Hao Z, Hampel B, Yagita H, Rajewsky K . T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med 2004; 199: 1355–1365.
Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 2004; 38: 176–181.
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y et al. Lethal effect of the anti-Fas antibody in mice. Nature 1993; 364: 806–809.
Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin ME . Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 2008; 7: 598–605.
Schwarz T . No eczema without keratinocyte death. J Clin Invest 2000; 106: 9–10.
Xu B, Bulfone-Paus S, Aoyama K, Yu S, Huang P, Morimoto K et al. Role of Fas/Fas ligand-mediated apoptosis in murine contact hypersensitivity. Int Immunopharmacol 2003; 3: 927–938.
Farley SM, Dotson AD, Purdy DE, Sundholm AJ, Schneider P, Magun BE et al. Fas ligand elicits a caspase-independent proinflammatory response in human keratinocytes: implications for dermatitis. J Invest Dermatol 2006; 126: 2438–2451.
Sancho-Martinez I, Martin-Villalba A . Tyrosine phosphorylation and CD95: a FAScinating switch. Cell Cycle 2009; 8: 838–842.
Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol 2009; 11: 1355–1362.
Leverkus M, Trautmann A . CD95-mediated signals in the skin: going out with an (inflammatory) bang? J Invest Dermatol 2006; 126: 2364–2366.
Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM . Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J Biol Chem 1997; 272: 25783–25786.
Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994; 76: 969–976.
Watanabe D, Suda T, Hashimoto H, Nagata S . Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J 1995; 14: 12–18.
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008; 13: 235–248.
Bagnoli M, Canevari S, Mezzanzanica D . Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol 2010; 42: 210–213.
Stratis A, Pasparakis M, Markur D, Knaup R, Pofahl R, Metzger D et al. Localized inflammatory skin disease following inducible ablation of I kappa B kinase 2 in murine epidermis. J Invest Dermatol 2006; 126: 614–620.
Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C et al. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci 2007; 120 (Part 8): 1480–1490.
Acknowledgements
This work was supported by a grant from the Medical Faculty, University of Cologne for AH (Koeln-Fortune program), by the Center for Molecular Medicine, University of Cologne and by Deutsche Forschungsgemeinschaft (SFB-829). We are grateful to Jonathan Howard, University of Cologne, for providing interferon-γ and to Gian Paolo Marcuzzi for supporting mouse work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by W Declerq
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Hedrych-Ozimina, A., Behrendt, K., Hao, Z. et al. Enhanced contact allergen- and UVB-induced keratinocyte apoptosis in the absence of CD95/Fas/Apo-1. Cell Death Differ 18, 155–163 (2011). https://doi.org/10.1038/cdd.2010.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2010.83
Keywords
This article is cited by
-
Necroptosis-mediated HMGB1 secretion of keratinocytes as a key step for inflammation development in contact hypersensitivity
Cell Death Discovery (2022)
-
Trehalose, sucrose and raffinose are novel activators of autophagy in human keratinocytes through an mTOR-independent pathway
Scientific Reports (2016)
-
Epidermal RelA Specifically Restricts Contact Allergen–Induced Inflammation and Apoptosis in Skin
Journal of Investigative Dermatology (2014)
-
Role of WWOX and NF-κB in lung cancer progression
Translational Respiratory Medicine (2013)