Abstract
Background:
Sox11 is a transcription factor expressed in foetal and neoplastic brain tissue, including gliomas. It has been shown to suppress the tumourigenicity of glioma stem cells in vivo, thereby being hypothesised to function as a tumour suppressor.
Methods:
We investigated the expression of Sox11 in 132 diffuse astrocytomas in relation to the regulator cell marker nestin, c-Met and IDH1-R132H, which have shown to be differentially expressed among the molecular subgroups of malignant gliomas, as well as to an inducer of astrocytic differentiation, that is, signal transducer and activator of transcription (p-STAT-3), clinicopathological features and survival.
Results:
Sox11 immunoreactivity was identified in all tumours irrespective of grade, but being correlated with p-STAT-3. Three out of seven cases showed partial Sox11 promoter methylation. In >50% of our cases neoplastic cells coexpressed Sox11 and nestin, a finding further confirmed in primary glioblastoma cell cultures. Furthermore, nestin, c-Met and IDH1-R132H expression differed among grade categories. Cluster analysis identified four groups of patients according to c-Met, nestin and IDH1-R132H expression. The c-Met/nestin high-expressor group displayed a higher Sox11 expression. Sox11 expression was an indicator of favourable prognosis in glioblastomas, which remained in multivariate analysis and validated in an independent set of 72 cases. The c-Met/nestin high-expressor group was marginally with shorter survival in univariate analysis.
Conclusions:
We highlight the importance of Sox11 expression as a favourable prognosticator in glioblastomas. c-Met/nestin/IDH1-R132H expression phenotypes recapitulate the molecular subgroups of malignant glioma.
Similar content being viewed by others
Main
Glioblastoma (astrocytoma grade IV) ranks as the most common and most aggressive primary brain tumour in adults (Maher et al, 2001), its prognosis remaining uniformly fatal despite concerted efforts and advances in surgery, radiotherapy and chemotherapy (including temozolomide; Stupp et al, 2005). The low patient-survival rate and the lack of robust treatment options have propelled a search for markers, which could identify subgroups of patients likely to benefit from molecularly targeted therapies.
The tumour stem cell concept pertains to the existence of small populations of tumourigenic neoplastic cells with self-renewal capacity (Clarke et al, 2006). Glioma stem cells (GSCs) are remarkably similar to normal neural stem cells in terms of expression of neural stem/progenitor cell markers, and upon induction differentiate into neuronal or glial lineages (Chen et al, 2012). Glioma stem cells are notorious for their resistance to radio/chemotherapy owing to activation of DNA repair pathways mediated by CHK1/CHK2 (Bao et al, 2006). Although the most accredited stem cell marker is CD133, its reliability has been questioned by the observations that tumourigenicity is not restricted to CD133+ tumour compartment (Beier et al, 2007) and that CD133−/nestin+ isolated glioma cells are able to grow tumours in rodents (Wang et al, 2008). Nestin is an intermediate filament the expression of which in normal adult brain is restricted to the subventricular zone and the dentate gyrus where normal neural precursors reside (Dictus et al, 2007). However, nestin is not a definite marker of GSCs being also expressed by neural/glial progenitor cells (Chen et al, 2012). It is therefore best regarded as a marker of dedifferentiated state.
Sox11 belongs to a group of transcription factors within the high mobility group (HMG) box protein superfamily, characterised by high sequence homology within their DNA-binding HMG domain (Wegner, 1999). High mobility group serves two functions, namely DNA binding and partner selection, which allow the selective recruitment of Sox proteins to specific promoters and transcription factors (Wilson et al, 2002). Sox genes are divided into eight groups and their sequential expression regulates neurogenesis from the early to the late stages (reviewed in Bergsland et al, 2011). In particular, SoxC group (to which Sox11 belongs) is responsible for the induction and the early phases of neuronal gene expression, whereas SoxB1 (to which the stem cell marker Sox2 belongs) maintains the progenitor stage of neural cells, counteracting neuronal differentiation (reviewed in Bergsland et al, 2011). Sox11 is mainly involved in neural development and organogenesis during foetal life, being absent from most normal adult tissues (reviewed in Penzo-Mendez, 2010). Recently, Sox11 has been advanced as a contributor to the pathogenesis of mantle cell lymphoma (Nygren et al, 2012), ovarian carcinoma (Brennan et al, 2009) and malignant gliomas (Weigle et al, 2005). Importantly, a link between GSCs and Sox11 is provided by a recent experimental study, documenting that GSCs are largely Sox11-negative and exogenous overexpression of Sox11 suppresses their tumourigenicity by inducing neuronal differentiation (Hide et al, 2009).
Microarray analyses have revealed subclasses of glioblastoma identified by congruence of genomic features that would otherwise be indistinguishable by morphology (reviewed in Chen et al, 2012). Although the number and types of subclasses vary depending on the sample pool and the methodology, the presence of proneural (PN) and mesenchymal (MES) subtypes has been confirmed by two independent studies (Phillips et al, 2006; Verhaak et al, 2010). These molecular subclasses differ with regard to survival rate (Colman et al, 2010; Vitucci et al, 2011), response to conventional therapies (Verhaak et al, 2010) and expression of stem and precursor cell markers, such as nestin (Colman et al, 2010). The MES subtype displays expression of MES markers such as MET (Phillips et al, 2006; Verhaak et al, 2010), whereas a major feature of the PN type are point mutations in isocitrate dehydrogenase (IDH)-1 gene, resulting in the expression of the mutant protein IDH1-R132H (Verhaak et al, 2010; Chen et al, 2012).
This study focuses on Sox11 expression in diffuse astrocytic tumours for which published information is limited to experimental data (Hide et al, 2009) and is organised in three parts. In the first part, we analysed the immunohistochemical expression of Sox11 in relation to standard clinicopathological parameters and survival using univariate analysis, and searched for Sox11 promoter methylation in the few cases with diminished or absent immunoreactivity. In the second part, we dealt with the relationships with nestin and the transcription factor, signal transducer and activator of transcription (p-STAT-3). The former was included because experimental data indicates that bona fide GSCs lack Sox11 while expressing nestin (Hide et al, 2009), but also because of its recently emphasised role in glioblastoma tumourigenesis (Lu et al, 2011). This relationship was investigated at the immunohistochemical but also at the flow cytometry level. The hallmark of glioblastoma p-STAT-3 (Brantley et al, 2008) promotes astrocytic differentiation (Rajan et al, 1998) and as such its expression may parallel that of Sox11. In the third part, we used three markers, namely nestin, c-Met and IDH1-R132H, which are differentially expressed among the molecular subclasses of glioblastoma (Verhaak et al, 2010) to segregate our cases into phenotypic subgroups analogous to the molecular subgroups. The ultimate goal was to examine whether Sox11 expression may vary according to the phenotypic subgroup of astrocytic tumours and may retain its prognostic utility in a multivariate analysis, including conventional prognosticators and the phenotypic subgroups. Finally, the prognostic effect of Sox11 was validated in an independent set of 72 glioblastoma patients.
Materials and methods
Patients’ description
This is a study of 132 adult patients with supratentorial diffuse infiltrating astrocytomas (grades II–IV) for whom archival primary tumour material at diagnosis, before radiotherapy, was available. Patients had been diagnosed in the First Department of Pathology, Laikon Hospital, National and Kapodistrian University of Athens, and treated as well as followed-up in Evangelismos, Asklepeion and Metropolitan Hospitals between 1999 and 2008. In all cases, the diagnoses and grading were peer-reviewed according to the principles laid down in the latest World Health Organization (WHO) Classification (Louis et al, 2007). Informed consent was obtained from all patients and the study was approved by the University of Athens Medical School Ethics Committee. The demographic data of our patients are summarised in Table 1.
Immunohistochemical staining
Immunostaining was performed on paraffin-embedded 4 μm sections of formalin-fixed tumour tissue using the two-step peroxidase-conjugated polymer technique (DAKO Envision kit, DAKO, Carpinteria, CA, USA). The primary antibodies used are listed in Table 2.
Sox11 immunostaining was available in the entire cohort (132 cases), IDH1-R132H and nestin in 111 cases, c-Met staining in 92 cases and p-STAT-3 in 113 cases. Evaluation of IDH1-R132H, nestin, c-Met and p-STAT-3 staining was performed by one pathologist, without knowledge of the clinical information. Sox11 immunostaining was evaluated by using the computerised image analysis software Image Pro software v5.1 (Media Cybernetics Inc., Rockville, MD, USA) on a Pentium III PC (Intel, Hillsboro, OR, USA), which evaluated both expression and staining intensity. In each case, immunoreactivity was calculated in at least 500 tumour cells counted in several randomly chosen high power fields. As Sox11 is known to be absent in normal brain tissue, any level of nuclear immunoreactivity was recorded as positive. Validation of automated quantification was performed in 20 random cases in which Sox11 nuclear immunoreactivity was manually estimated. IDH1-R132H was recorded as positive when cytoplasmic staining of tumours was seen (Hartmann et al, 2010). Regarding c-Met expression, tumours were assigned into low expression (no immunoreactivity/low expression levels or staining intensity similar to that of normal brain tissue) or high expression group (moderate or strong immunoreactivity in >25% of tumour cells), as proposed by Kong et al (2009). Nestin intensity was in all positive cases strong. Therefore, only the percentage of positive cells was taken into account for statistical analysis. The cut-off for high vs low nestin expression was 30%, as previously suggested (Rushing et al, 2010). These thresholds were evaluated using receiver operating characteristics (ROC) curves. For p-STAT-3 both the percentage of positive neoplastic nuclei and staining intensity was recorded, and a Histo-score (H-score) based on the percentage of stained neoplastic cells multiplied by staining intensity was calculated.
Human primary glioblastoma cell cultures
Three fresh glioblastoma samples were obtained in collection medium (Dulbecco’s modified Eagle’s medium/Ham’s F12, penicillin and streptomycin, amphotericin B), and primary glioblastoma cell cultures were established, as previously described (Zisakis et al, 2007). When cell cultures reached confluency, flow cytometric analyses for nestin and Sox11 expression was performed.
Flow cytometry analysis
At least 4 × 106 cells were used for flow cytometric analysis. Cells were fixed in 1% paraformaldehyde (Sigma, St. Louis, MO, USA) for 10 min at room temperature. Fc receptors were blocked using 5% FBS (Invitrogen, Antisel SA, Greece) in PBS (Invitrogen). After washing, the cells were permeabilised in PBS supplemented with 0.5% FBS and 0.1% Triton-X-100 (Sigma). The antibodies used are listed in Table 2. Isotype-specific antibodies were used as negative controls. Fluoresence was measured on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analysis of the data was performed using CellQuest software (BD Biosciences). The percentages of positive cells were calculated by the subtraction of the background from control stainings.
Methylation-sensitive high-resolution melting analysis sequencing
In order to detect the promoter methylation status of Sox11 gene, we used a real-time PCR approach, followed by high-resolution melting curve analysis. This is considered as a rapid, highly sensitive and efficient method displaying the sequence-dependent melting profile of an amplicon on a Light Cycler 480 (Roche Diagnostics, GmbH, Mannheim, Germany) in one single run. All sodium bisulphite-treated DNAs were analysed in triplicate. PCR products were also analyzed by Sanger sequencing to identify individual methylated CpGs, as previously described (Gustavsson et al, 2010).
Statistical analysis
Statistical analysis was performed by an MSc Biostatistician (GL). In the basic statistical analysis, Sox11 and p-STAT-3 were treated as continuous variables. c-Met and nestin expression were treated as dichotomous variables, that is, low or high, as already described. Associations of the molecules under study with clinicopathological parameters were tested using non-parametric tests, with correction for multiple comparisons (Kruskal-Wallis ANOVA, Mann-Whitney U-test, Fisher’s exact test and Spearman’s rank correlation coefficient, as appropriate).
Hierarchical cluster analysis was performed using the Ward method in order to classify our cases into subgroups on the basis of their differential expression of c-Met, nestin and IDH1-R132H. The dissimilarity between observations was evaluated using the Jaccard method.
Survival analysis was performed using death-by-disease as end point. The effect of various clinicopathological parameters (age, sex, tumour location, radiotherapy, chemotherapy, extent of surgical resection and histological grade) as well as Sox11, c-Met, nestin and IDH1-R132H immunoreactivity on clinical outcome was assessed by plotting survival curves according to the Kaplan-Meier’s method and comparing groups using the log-rank test or the Wilcoxon Breslow test, as appropriate. p-STAT-3 was not included in survival analysis because its prognostic significance has been dealt with in a previous study of our group (Piperi et al, 2011) Numerical variables were categorised on the basis of cut-off values provided by ROC curves. Power estimation of the log-rank tests regarding Sox11 expression was performed using the Freedman method for estimation of censored data. Multivariate analysis was performed using Cox’s proportional hazard estimation model in order to evaluate the predictive power of each parameter independently of others. To avoid any ‘data-driven’ categorisation, numerical variables (age, Sox11 labelling index) were entered in multivariate analysis in continuous form.
Statistical calculations were performed using the statistical package STATA 11.0 for Windows (College station, TX, USA). Cluster analysis and subsequent figures were performed using R 2.12.0 for Windows (CRAN, Vienna, Austria). All results with a two-sided P level ⩽0.05 were considered statistically significant.
Validation cohort
An independent set of patients with glioblastomas was used to validate the chosen cut-off values for the expression of Sox11 in univariate analysis. The results of univariate survival analysis for Sox11 expression in the population group were used to calculate the required number of patients in the validation group for an adequately powered analysis (90%). In order to detect a hazard ratio (HR) of 0 348, as calculated in the patients’ group using a two-sided log-rank test, and to achieve 90% power at a 0.05 significance level, 58 patients would be needed. The validation group we used consisted of 72 patients, the demographic data of whom are shown in Table 1. These patients were diagnosed and treated at Red-Cross Hospital between 2007 and 2011.
Results
Sox11 expression in astrocytic tumours associations with clinicopathological features and analysis of Sox11 promoter methylation status
Sox11 immunoreactivity was nuclear and was observed in all cases in the population cohort and in 67 out of 72 cases of the validation cohort, ranging from 0.5% to 94%. Endothelial cells of tumoural vessels were also positive for Sox11, and therefore served as internal positive controls (Table 3; Figure 1). Normal brain did not display any Sox11 immunoreactivity, either in normal astrocytes or in endothelial cells. Although the staining intensity varied among tumours from moderate to strong, all the analyses regarding Sox11 immunoexpression were based on the percentage of positive cells, according to preliminary statistical analysis of our cohort, indicating that staining intensity did not correlate with any parameter in this investigation and the respective literature concerning other neoplasms (Ek et al, 2008; Brennan et al, 2009; Gustavsson et al, 2010; Nygren et al, 2012). Automatically and manually estimated labelling indices were strongly correlated (ρ=0.9743) and directly comparable as the P value of detecting a difference >5% between the two measurements was <0.0001 (Wilcoxon-matched paired one-sided test). Sox11 expression was not correlated with tumour histological grade (Kruskal-Wallis ANOVA, P>0.10, Figure 2A) or any other clinicopathological features.
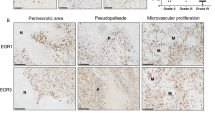
(A, B) Immunohistochemical expression of Sox11 in a grade-II diffuse astrocytoma (A) and a grade-IV (B) glioblastoma. (C, D) Immunohistochemical expression of Sox11 (C) and nestin (D) at the same region in a glioblastoma case. Both proteins were abundantly expressed by the majority of the neoplastic cells. (E, F) High Sox11 (E) and moderate nestin. Nestin (F) expression at the same region in a diffuse astrocytoma case. Many of the cells expressing Sox11 were also positive for nestin.
Seven samples displaying different levels of Sox11 expression (ranging between 0 and 95%) were analysed for Sox11 promoter methylation status. Cases showing reduced (<40%) or absent Sox11 immunoexpression displayed at least partially methylated promoter sequences, whereas those having enhanced Sox11 expression (>60%) displayed unmethylated promoters (Figure 3).
Nestin, c-Met, IDH1-R132H expression in astrocytic tumours and associations with clinicopathological features
Nestin and c-Met expression was cytoplasmic and observed in 85.6% (95/111) and 46.7% (43/92) of the examined cases, respectively, (Table 3). The IDH1-R132H immunostaining was cytoplasmic and was evaluated as positive or negative, as described previously (Hartmann et al, 2010). None of the IDH1-R132H-positive cases displayed high c-Met expression (Fisher’s exact test, P=0.0030). Accordingly, all but three cases positive for IDH1-R132H displayed a lower nestin expression (<30%; Fisher’s exact test, P<0.0001).
c-Met and nestin expression were higher in glioblastomas when compared with diffuse and anaplastic astrocytomas (Fischer’s exact test, P=0.030 and P=0.001, respectively), whereas IDH1-R132H immunoreactivity was mostly seen in grades II/III (Fischer’s exact test, P=0.0250). All other associations with clinicopathological features were not significant (P>0.10).
Associations between Sox11 and nestin, c-Met, IDH1-R132H or p-STAT-3 expression
Sox11 expression was positively correlated with p-STAT-3 H-score and nestin expression (ρ=0.3705, P=0.0001 and ρ=0.3029, P=0.012, respectively). Interestingly, in 58.55% (65 out of 111) of the examined cases, the sum of Sox11 and nestin labelling indices exceeded 100% by a median of 29% (range 2%–72%), clearly denoting coexpression of the two molecules by at least a subset of neoplastic cells. Two such cases with significant coexpression are shown in Figure 1. No significant relationship between Sox11 and c-Met or IDH1-R132H was documented (P>0.10).
Coexpression of Sox11 and nestin in a subset of primary glioma cells
Flow cytometric analysis was used to verify Sox11 and nestin’s coexpression in three primary glioblastoma cell cultures. Representative data of single and double staining analysis for Sox11 and nestin are shown in Figure 4. Single staining revealed that both proteins are independently expressed in primary glioblastoma cells, with nestin expression separating the culture into two distinct populations. Furthermore, double staining revealed a subset population of 9.14% of gated glioma cells to be positive for both Sox11 and nestin (Figure 4).
Flow cytometric analysis of Sox11 and nestin expression in glioblastoma cell cultures. Unstained glioblastoma cells were used for gating (R1) of the cell population for analysis (A). Single staining of nestin (B, D) and Sox11 (C, E) expression is shown in histograms and dot plots, respectively. Isotype-specific negative control is depicted in green. Double staining of glioma cells for nestin and Sox11 revealed a subpopulation of 9.14% of gated cells being positive for both proteins (F). The bar graph presents the fraction of positive cells (mean values) for Sox11 and/or nestin staining in the three primary glioblastoma culture (G).
Classification of cases into subgroups according to nestin, c-Met and IDH1-R132H expression
Hierarchical cluster analysis was performed using IDH1-R132H, c-Met and nestin expression. The dendrogram of this analysis is presented in Figure 5. According to this dendrogram, four groups consisting of cases with similar expression patterns were identified. The first group included 17 IDH1-R132H-negative cases overexpressing c-Met, 76.5% (13 out of 17) of which displayed also high levels of nestin. All cases in the second group (18 patients) overexpressing nestin were negative for IDH1-R132H and displayed low levels of c-Met expression. Accordingly, all cases in the third group (18 patients) were positive for IDH1-R132H and displayed low levels of nestin and c-Met expression. The remaining 17 patients of the fourth group neither overexpressed c-Met/nestin nor expressed IDH1-R132H. When this analysis was repeated in glioblastomas, the same four subgroups emerged.
Interestingly, Sox11 expression was marginally higher in the first two subgroups characterised by the presence of c-Met and/or nestin overexpression (Mann-Whitney U test, P=0.0548, Figure 2B). Although Sox11 expression seemed to be higher in the first subgroup characterised by c-Met overexpression than in the third group represented by IDH1-R132H-positive cases, this difference failed to attain statistical significance (P>0.10).
Survival analysis
Univariate survival analysis was carried out in grades II/III and in glioblastomas separately. The results are presented in Table 4. Sox11 overexpression was correlated with improved overall survival in glioblastomas (P=0.0002, Figure 6A), irrespective of or despite its coexpression with nestin (log-rank test, P=0.2756). The comparison of survival functions among the groups allocated by Sox11 expression in glioblastomas had a statistical power of 0.9871 at a significance level of 0.05. In the entire cohort, there was a trend for favourable survival for those cases displaying IDH1-R132H positivity (Wilcoxon Breslow test, P=0.0736, Figure 6C), which, however, was not reproduced when grades II/III and IV were analysed separately (P>0.10). Interestingly, there was an almost significant decreased probability of survival in the first two subgroups of patients characterised by c-Met and/or nestin overexpression in grades II/III (P=0.0505). The worse behaviour of these expression subgroups is illustrated in Figure 6D.
Multivariate survival analysis, including all parameters for the patients for whom staining results for the molecules under study were available, is presented in Table 5. Sox11 expression emerged as an independent prognosticator (HR=0.975, P=0.009), along with histological grade, patients’ age and the administration of radiotherapy. Importantly, Sox11 expression prognostic significance remained when analysis was restricted to glioblastomas (HR=0,339, P=0.010).
Survival analysis-validation group
The overall survival was significantly lower in the Sox11 low-expressor compared with the Sox11 high-expressor group (log-rank test, P<0.0001, Figure 6B). This effect remained after adjustment for patient’s age and treatment (HR=0.27, P<0.0001), thus corroborating the respective findings established in the patients’ cohort.
Discussion
Since their discovery in 2006, GSCs have been advanced as the primary ‘culprit’ in the inherent resistance of glioblastoma to chemo/radiotherapy and the invariable maintenance of residual disease after conventional therapies (Kim et al, 2011). Although the tumour stem cell hypothesis has been challenged because of the lack of reliable stem cell markers, it has certainly revolutionised our understanding of antineoplastic therapy. Eliminating this minority cell population either by blocking components of pathways or ‘stemness’ factors that sustain its growth, or by upregulating the expression of factors that promote differentiation might prove a more biologically rational and efficient approach for the eradication of tumours, such as glioblastoma, resistant to standard therapies (Kim et al, 2011).
The vast majority of our diffuse astroglial tumours exhibited Sox11 immunoreactivity. The pattern of staining was nuclear, as reported by other investigators in foetal brain tissue (Haslinger et al, 2009), mantle cell lymphoma (Nordström et al, 2012), ovarian carcinoma (Brennan et al, 2009) and medulloblastoma (de Bont et al, 2008), conforming to the nuclear localisation signals conserved among all Sox proteins. Indeed, the lack of nuclear staining reportedly correlates with the absence of Sox11 mRNA in normal and neoplastic tissues (Ek et al, 2008). The abundant expression of Sox11 in our cases is in harmony with the results of primary glioblastoma cell cultures and those obtained by quantitative real-time PCR revealing a 5- to >600-fold Sox11 mRNA overexpression in malignant gliomas (Weigle et al, 2005). The absence of Sox11 immunoreactivity in adjacent normal brain tissue along with its rather uniform distribution of its levels among low -and high- grade cases lends support to the assumption that, following downregulation in normal adult brain, Sox11 is reactivated early in gliomagenesis, as a result of dedifferentiation (Weigle et al, 2005). In keeping with this interpretation, de Bont et al (2008) noticed more prominent staining in medulloblastoma than that in ependymoma. Therefore, Sox11 might also be of potential value as a diagnostic marker of neoplastic astrocytes. A similar absence of correlation between Sox11 expression and histological grade has been noted in ovarian carcinoma (Sernbo et al, 2011), as opposed to meningiomas where marked upregulation of Sox11 mRNA has been observed in grade III (Stuart et al, 2011).
Despite the upregulation of Sox11 mRNA in some tumours as alluded to, the molecular mechanisms by which Sox11 modulates oncogenesis are largely unknown and somewhat controversial (Penzo-Mendez, 2010). It has been hypothesised that Sox11 may contribute to the pathogenesis of mantle cell lymphoma by regulating genes involved in cell proliferation and apoptosis (Klein and Assoian 2008). Also, in a neuroblastoma cell line, knockdown of Sox11 augmented the expression of the proapoptotic gene BNIP3 and decreased the expression of the antiapoptotic gene TANK (Jankowski et al, 2006). It is of interest that Sox4, which shares considerable sequence homology to Sox11, harbours both proapoptotic and antiapoptotic properties, its function being tissue specific and dependent on external signals (Penzo-Mendez, 2010).
Perhaps of even greater clinical relevance is our observation that increased levels of Sox11 nuclear expression were associated with a more favourable clinical outcome in both univariate and multivariate analysis. To the best of our knowledge, this is the first attempt to delineate the potential utility of Sox11 protein expression as a prognostic factor in astrocytic gliomas. A similar favourable prognostic significance was assigned to Sox11 expression in ovarian carcinoma in terms of recurrence-free survival (Brennan et al, 2009; Sernbo et al, 2011), whereas in mantle cell lymphomas its prognostic significance has been disputed (Ek et al, 2008). Several lines of experimental evidence attest to the tumour suppressor function of Sox11 in tumours. First, Sox11 is epigenetically silenced through methylation of CpG islands in B cell lymphomas, other than mantle cell lymphomas, and ovarian carcinomas, this being a common mechanism for silencing of tumour suppressor genes (Brennan et al, 2009; Gustavsson et al, 2010). Our results showing that reduced Sox11 expression may be because of the presence of promoter methylation supports the possibility of an epigenetic control of Sox11 expression also in a subset of astrocytic gliomas. Second, Sox11 overexpression in B-cell lymphoma cell lines exerts an inhibitory effect on proliferation involving TGF-β and components of the Rb-E2F pathway, as identified by global gene expression analysis (Gustavsson et al, 2010). Third, constitutive Sox11 knockdown in a mantle-cell lymphoma cell line promotes a highly proliferative behaviour in vivo (Conrotto et al, 2011). Fourth, experiments in the induced mouse glioma cell line NSCL61 have disclosed that overexpression of Sox11 blocked their tumourigenicity and recurrent glioblastomas, which are enriched in GSCs were Sox11-negative. In addition, downregulation of Sox11 mRNA resulted in diminished patient survival (Hide et al, 2009). Interestingly, Sox4 is also a favourable prognostic marker in bladder cancer (Aaboe et al, 2006) and medulloblastoma (de Bont et al, 2008).
A significant positive relationship was documented between Sox11 and p-STAT-3 expression. The signal transducer and activator of transcription (p-STAT-3) is known to promote neurite growth (Moore et al, 2011) and induces astrocytic differentiation during central nervous system (CNS) development (Rajan et al, 1998). Therefore, the relationship between these two transcription factors may reflect their functional similarity. It is worthy of note that silencing of Sox11 in mantle-cell lymphoma cell lines caused a dysregulation of STAT-1 transcription (Conrotto et al, 2011). Whether a similar interaction may exist between Sox11 and STAT-3 is presently unknown.
An intriguing finding emerging from the present investigation is the coexpression of nestin and Sox11 by a subset of neoplastic cells (as high as 72%) in almost 60% of our cases, and corroborated by flow cytometry in three primary glioblastoma cell cultures. The percentage may be even higher as the presence of a few cells double positive for both nestin and Sox11 in the remaining cases cannot be ruled out. Given that the former is a marker of undifferentiated cells, whereas the latter a transcription factor promoting differentiation of neural precursors, this finding seems paradoxical and at variance with the reported inhibition of nestin, following forced upregulation of Sox11 in the induced mouse glioma cell line NSCL61 (Hide et al, 2009). At present, it is not clear whether these double-positive nestin and Sox11 cells are GSCs or glial precursors. However, immunohistochemistry in embryonic spinal cord sections has clearly shown that the two proteins are not mutually exclusive as there exist two populations of nestin-expressing cells in foetal CNS, that is, those expressing SoxB1 and those expressing Sox11 (Tanaka et al, 2004). Our findings may be explained by the fact that Nestin gene expression in the early neural tube and possibly in neural primordial cells, is driven by the synergic interaction between group B1/C Sox and Class III POU (Pit-Oct-Unc) transcription factors, binding sites for which are found on Nestin’s enhancer (Tanaka et al, 2004). What emerges from our data is that the favourable prognostic effect of Sox11 cannot be with certainty attributed to its absence from GSCs, as originally suggested (Hide et al, 2009), as cases coexpressing Sox11 and nestin are not significantly different from those with Sox11-positivity in terms of survival.
Dissecting our cases by the use of cluster analysis according to nestin, c-Met and IDH1-R132H expression yielded four groups. The first group characterised by high c-Met expression might correspond to the MES subclass identified by gene expression analysis (Phillips et al, 2006; Verhaak et al, 2010). The second group characterised by high nestin expression without high c-Met is analogous to the classical subtype identified by Verhaak et al (2010). The third group including all IDH1-R132H-positive cases clearly represents the PN subtype (Phillips et al, 2006; Verhaak et al, 2010) and the fourth group included all the remaining cases. Whether this group might be related to the neural subtype proposed by Verhaak et al (2010) remains to be seen. It is noteworthy that high c-Met or nestin expression was mutually exclusive with IDH1-R132H, reflecting the striking mutual exclusivity of the PN and MES gene signatures (Verhaak et al, 2010). These three molecules’ expression significantly differed across the various grade categories with high c-Met and nestin expression prevailing in glioblastomas and IDH1-R132H positivity in grades II/III, as expected (Kong et al, 2009; Hartmann et al, 2010; Wan et al, 2011). However, only nestin marginally correlated with survival in grades II/III in univariate analysis. Although IDH1-R132H positivity implied a marginally improved survival in the entire cohort, we failed to reproduce the expected favourable effect when various grades were analysed separately. According to a number of most recently published studies, the prognostic value of IDH1 mutation status is mostly seen in grades III and IV, whereas in grade II it is not substantiated as a prognostic factor (Kim et al 2010; Mukasa et al 2012; Boots-Sprenger et al 2013). However, it has been claimed that the prognostic significance of IDH1 mutations is lost in glioblastoma patients older than 50 years (Boots-Sprenger et al 2013), who comprise 71% of our glioblastoma patient population, this probably accounting for our inability to substantiate the prognostic utility of IDH1-R132H in our series. When the four expression groups were introduced into Cox’s model, including age, grade, Sox11 and treatment, their significance disappeared, Sox11 being more informative in this regard.
We also observed that Sox11 expression was more pronounced within the c-Met/ nestin-overexpressing groups. This is consistent with the reported Sox11 upregulation in early progenitor human multipotent stromal cells (Larson et al, 2010) and in the MES/undifferentiated phenotype of serous ovarian carcinoma associated with the transcription of developmentally associated factors (Tothill et al, 2008).
It should be borne in mind that the expression phenotypes derived by cluster analysis in our investigation are not meant to discount the importance of the molecular categorisation of gliomas. However, they reinforce the use of key markers that have proved pertinent to the molecular subclasses for the immunohistochemical identification of biologically meaningful groups of astroglial tumours.
In conclusion, we herein highlight for the first time the importance of Sox11 tissue expression as an independent marker of favourable significance, strengthening its tumour suppressor function in astroglial tumours. Given the paucity of favourable prognostic markers in these tumours, our data may provide a rationale for the therapeutic incorporation of Sox11 upregulation in those cases with low expression levels secondary to promoter methylation, by the use of demethylating agents. Our investigation also adds to the growing consensus that astroglial tumours comprise distinct molecular subtypes, which to some extent may be recapitulated at the tissue level in the c-Met/nestin/IDH1-R132H expression groups. The latter also differ with regard to Sox11 expression. Although at present the identification of these expression subgroups may be of lesser prognostic significance, it is anticipated that it may be of value in the development of personalised therapies for patients with these tumours.
Change history
28 May 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aaboe M, Birkenkamp-Demtroder K, Wiuf C, Sørensen FB, Thykjaer T, Sauter G, Jensen KM, Dyrskjøt L, Ørntoft T (2006) SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization. Cancer Res 66: 3434–3442
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP (2007) CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differentiatial growth characteristics and molecular profiles. Cancer Res 67: 4010–4015
Bergsland M, Ramsköld D, Zaouter C, Klum S, Sandberg R, Muhr J (2011) Sequentially acting Sox transcription factors in neural lineage development. Genes Dev 25: 2453–2464
Boots-Sprenger SH, Sijben A, Rijntjes J, Tops BB, Idema AJ, Rivera AL, Bleeker FE, Gijtenbeek AM, Diefes K, Heathcock L, Aldape KD, Jeuken JW, Wesseling P (2013) Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol e-pub ahead of print 22 February 2013 doi:10.1038/modpathol.2012.166
Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K, Roarty K, Benveniste EN (2008) Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res 14: 4694–4704
Brennan DJ, Ek S, Doyle E, Drew T, Foley M, Flannelly G, O’Connor DP, Gallagher WM, Kilpinen S, Kallioniemi OP, Jirstrom K, O’Herlihy C, Borrebaeck CA (2009) The transcription factor Sox11 is a prognostic factor for improved recurrence-free survival in epithelial ovarian cancer. Eur J Cancer 45: 1510–1517
Chen J, McKay RM, Parada LF (2012) Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149: 36–47
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM (2006) Cancer stem cells--perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res 66: 9339–9344
Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, Popoff S, Nutt CL, Louis DN, Cairncross JG, Gilbert MR, Phillips HS, Mehta MP, Chakravarti A, Pelloski CE, Bhat K, Feuerstein BG, Jenkins RB, Aldape K (2010) A multigene predictor of outcome in glioblastoma. Neuro Oncol 12: 49–57
Conrotto P, Andréasson U, Kuci V, Borrebaeck CA, Ek S (2011) Knock-down of SOX11 induces autotaxin-dependent increase in proliferation in vitro and more aggressive tumors in vivo. Mol Oncol 5: 527–537
de Bont JM, Kros JM, Passier MM, Reddingius RE, Sillevis Smitt PA, Luider TM, den Boer ML, Pieters R (2008) Differential expression and prognostic significance of SOX genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro Oncol 10: 648–660
Dictus C, Tronnier V, Unterberg A, Herold-Mende C (2007) Comparative analysis of in vitro conditions for rat adult neural progenitor cells. J Neurosci Methods 161: 250–258
Ek S, Dictor M, Jerkeman M, Jirström K, Borrebaeck CA (2008) Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood 111: 800–805
Gustavsson E, Sernbo S, Andersson E, Brennan DJ, Dictor M, Jerkeman M, Borrebaeck CA, Ek S (2010) SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Mol Cancer 9: 187
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120: 707–718
Haslinger A, Schwarz TJ, Covic M, Lie DC (2009) Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci 29: 2103–2114
Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu J, Kondo T (2009) Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res 69: 7953–7959
Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM (2006) SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 143: 501–514
Kim KJ, Lee KH, Kim HS, Moon KS, Jung TY, Jung S, Lee MC (2011) The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology 31: 494–502
Kim YH, Nobusawa S, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Nakazato Y, Tanaka Y, Vital A, Mariani L, Stawski R, Watanabe T, De Girolami U, Kleihues P, Ohgaki H (2010) Molecular classification of low-grade diffuse gliomas. Am J Pathol 177: 2708–2714
Klein EA, Assoian RK (2008) Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 121: 3853–3857
Kong DS, Song SY, Kim DH, Joo KM, Yoo JS, Koh JS, Dong SM, Suh YL, Lee JI, Park K, Kim JH, Nam DH (2009) Prognostic significance of c-Met expression in glioblastomas. Cancer 115: 140–148
Larson BL, Ylostalo J, Lee RH, Gregory C, Prockop DJ (2010) Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells. Tissue Eng Part A 16: 3385–3394
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97–109
Lu WJ, Lan F, He Q, Lee A, Tang CZ (2011) Inducible expression of stem cell associated intermediate filament nestin reveals an important role in glioblastoma carcinogenesis. Int J Cancer 128: 343–351
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15: 1311–1333
Moore DL, Goldberg JL (2011) Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol 71: 1186–1121
Mukasa A, Takayanagi S, Saito K, Shibahara J, Tabei Y, Furuya K, Ide T, Narita Y, Nishikawa R, Ueki K, Saito N (2012) Significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients. Cancer Sci 103: 587–592
Nordström L, Andréasson U, Jerkerman M, Dictor M, Borrebaeck C, Ek S (2012) Expanded clinical and experimental use of SOX-11 using a monoclonal antibody. BMC Cancer 12: 269
Nygren L, Baumgartner Wennerholm S, Klimkowska M, Christensson B, Kimby E, Sander B (2012) Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood 119: 4215–4223
Penzo-Méndez A (2010) Critical roles for SoxC transcription factors in development and cancer. Int J Biochem Cell Biol 42: 425–428
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9: 157–173
Piperi C, Samaras V, Levidou G, Kavantzas N, Boviatsis E, Petraki K, Grivas A, Barbatis C, Varsos V, Patsouris E, Korkolopoulou P (2011) Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine 55: 387–395
Rajan P, McKay RDG (1998) Multiple routes to astrocytic differentiation in the CNS. J Neurosci 18: 3620–3629
Rushing EJ, Sandberg GD, Horkayne-Szakaly I (2010) High-grade astrocytomas show increased nestin and Wilms’s tumor gene (WTI) protein expression. Inl J Surg Pathol 18: 255–259
Sernbo S, Gustavsson E, Brennan DJ, Gallagher WM, Rexhepaj E, Rydnert F, Jirström K, Borrebaeck CA, Ek S (2011) The tumour suppressor SOX11 is associated with improved survival among high grade epithelial ovarian cancers and is regulated by reversible promoter methylation. BMC Cancer 11: 405
Stuart JE, Lusis EA, Scheck AC, Coons SW, Lal A, Perry A, Gutmann DH (2011) Identification of gene markers associated with aggressive meningioma by filtering across multiple sets of gene expression arrays. J Neuropathol Exp Neurol 70: 1–12
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996
Tanaka S, Kamachi Y, Tanouch A, Hamada H, Jing N, Kondoh H (2004) Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol 8834–8846
Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, Traficante N, Fereday S, Hung JA, Chiew YE, Haviv I Australian Ovarian Cancer Study Group Gertig D, DeFazio A, Bowtell DD (2008) Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 14: 5198–5208
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17: 98–110
Vitucci M, Hayes DN, Miller CR (2011) Gene expression profiling of gliomas: merging genomic and histopathological classification for personalized therapy. BJC 104: 545–553
Wan F, Herold-Mende C, Campos B, Centner FS, Dictus C (2011) Association of stem cell-related markers and survival in astrocytic gliomas. Biomarkers 16: 136–143
Wang J, Sakariassen PØ, Tsinkalovsky O, Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PØ (2008) CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer 122: 761–768
Wegner M (1999) From head to toes: the multiple facets of Sox proteins. Nucl Acid Res 27: 1409–1420
Weigle B, Ebner R, Temme A, Schwind S, Schmitz M, Kiessling A, Rieger MA, Schackert G, Schackert HK, Rieber EP (2005) Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol Rep 13: 139–144
Wilson M, Koopman P (2002) Matching SOX: Partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev 12: 441–446
Zisakis A, Piperi C, Themistocleous MS, Korkolopoulou P, Boviatsis EI, Sakas DE, Patsouris E, Lea RW, Kalofoutis A (2007) Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine 39: 99–105
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Korkolopoulou, P., Levidou, G., El-Habr, E. et al. Sox11 expression in astrocytic gliomas: correlation with nestin/c-Met/IDH1-R132H expression phenotypes, p-Stat-3 and survival. Br J Cancer 108, 2142–2152 (2013). https://doi.org/10.1038/bjc.2013.176
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.176
Keywords
This article is cited by
-
SOX11 is a sensitive and specific marker for pulmonary high-grade neuroendocrine tumors
Diagnostic Pathology (2022)
-
SoxC transcription factors: multifunctional regulators of neurodevelopment
Cell and Tissue Research (2018)
-
Establishing cut-off points with clinical relevance for bcl-2, cyclin D1, p16, p21, p27, p53, Sox11 and WT1 expression in glioblastoma - a short report
Cellular Oncology (2018)
-
Nestin Expression Is Associated with Poor Clinicopathological Features and Prognosis in Glioma Patients: an Association Study and Meta-analysis
Molecular Neurobiology (2017)
-
The role of tumor suppressor gene SOX11 in prostate cancer
Tumor Biology (2015)