Abstract
Background:
Fatty acid synthase (FASN) is overexpressed and associated with poor prognosis in several human cancers. Here, we investigate the effect of FASN inhibitors on the metastatic spread and angiogenesis in experimental melanomas and cultured melanoma cells.
Methods:
The lung colonisation assay and cutaneous melanomas were performed by the inoculation of mouse melanoma B16-F10 cells in C57BL6 mice. Blood vessel endothelial cells (RAEC and HUVEC) were applied to determine cell proliferation, apoptosis, and the formation of capillary-like structures. Vascular endothelial growth factor A (VEGFA) expression was evaluated by quantitative RT–PCR and ELISA in B16-F10, human melanoma (SK-MEL-25), and human oral squamous carcinoma (SCC-9) cells. Conditioned media from these cancer cell lines were used to study the effects of FASN inhibitors on endothelial cells.
Results:
B16-F10 melanoma-induced metastases and angiogenesis were significantly reduced in orlistat-treated mice. Fatty acid synthase inhibitors reduced the viability, proliferation, and the formation of capillary-like structures by RAEC cells, as well as the tumour cell-mediated formation of HUVEC capillary-like structures. Cerulenin and orlistat stimulated the production of total VEGFA in B16-F10, SK-MEL-25, and SCC-9 cells. Both drugs also enhanced VEGFA121, 165, 189, and 165b in SK-MEL-25 and SCC-9 cells.
Conclusion:
FASN inhibitors reduce metastasis and tumour-induced angiogenesis in experimental melanomas, and differentially modulate VEGFA expression in B16-F10 cells.
Similar content being viewed by others
Main
Fatty acid synthase (FASN) (EC2.3.1.85) is responsible for the endogenous fatty acid production from acetyl-CoA and malonyl-CoA (Menendez and Lupu, 2007). Its expression and activity is downregulated in normal cells, except in the liver, lactating breast, foetal lung, and adipose tissue (Weiss et al, 1986; Kuhajda, 2000). Conversely, in several human malignancies, such as those of prostate, breast, ovary, oral cavity, melanoma, and soft tissue sarcomas, FASN is overexpressed (Pizer et al, 1996a, 1996b; Gansler et al, 1997; Alò et al, 2000; Swinnen et al, 2002; Innocenzi et al, 2003; Rossi et al, 2003; Takahiro et al, 2003; Visca et al, 2004; Kapur et al, 2005; Van de Sande et al, 2005; Rossi et al, 2006; Silva et al, 2008; Ogino et al, 2008; Dowling et al, 2009; da Silva et al, 2009; Walter et al, 2009; Ueda et al, 2010) and associated with poor prognosis (Pizer et al, 1996b; Gansler et al, 1997; Alò et al, 2000; Swinnen et al, 2002; Innocenzi et al, 2003; Rossi et al, 2003; Takahiro et al, 2003; Visca et al, 2004; Kapur et al, 2005; Van de Sande et al, 2005; Rossi et al, 2006; Ogino et al, 2008; da Silva et al, 2009; Walter et al, 2009; Ueda et al, 2010). Fatty acid synthase inhibition reduces cell proliferation, enhances apoptosis, decrease the size of prostate, ovarian, and breast cancer xenografts, and is chemopreventive in the Neu-N mouse model for breast cancer (Pizer et al, 1996a, 1996b; Furuya et al, 1997; Pizer et al, 1998; Li et al, 2001; Kridel et al, 2004; Alli et al, 2005; Zhou et al, 2007). Importantly, FASN was recently described as a metabolic oncogene in prostate cancer, as its forced expression transforms and confer tumorigenicity to immortalised prostate epithelial cells overexpressing androgen receptor (Migita et al, 2009). Despite the marginal role of FASN in normal cells, orlistat reduces endothelial cell proliferation and neovascularisation in an ex vivo assay, suggesting an antiangiogenic ability for this drug (Browne et al, 2006).
Orlistat is a pancreatic lipase inhibitor developed as anti-obesity drug, which also acts as an irreversible FASN inhibitor with antitumour properties (Kridel et al, 2004). Our prior studies show that orlistat reduces the proliferation and promotes apoptosis in the mouse melanoma cell line B16-F10 (Carvalho et al, 2008; Zecchin et al, 2011). These observations explain, at least in part, the significant decrease in the metastatic spread of the same cells in orlistat-treated mice with intraperitoneal melanomas (Carvalho et al, 2008). In addition, orlistat blocks cell cycle progression and promotes apoptosis through a PEA-3-mediated transcriptional repression of the Her2/Neu oncogene in Her2/Neu-overexpressing breast cancer cells (Menendez et al, 2005a). Moreover, FASN inhibition with C75, a synthetic analogue of cerulenin, upregulates vascular endothelial growth factor A (VEGFA) production, MAPK activation, and HIF-1α accumulation in Her2/Neu-overexpressing breast and ovarian cancer cells (Menendez et al, 2005b).
Vascular endothelial growth factor A is the main regulator of angiogenesis, generated as multiple isoforms grouped in pro- and anti-angiogenic families, both functioning mainly through the vascular endothelial cell receptor-2 (VEGFR-2; Harper and Bates, 2008). Vascular endothelial growth factor A production is low in benign nevi and progressively increased from dysplastic nevi to melanoma (Einspahr et al, 2007). Accordingly, the transition from radial to vertical growth phase in human melanomas is also characterised by increased VEGFA expression (Erhard et al, 1997). In a recent tissue microarray study including benign nevi, primary and metastatic melanomas, VEGFA, VEGFR-1, and -2 were found to be upregulated in the malignant tumours whereas VEGFR-2 positivity was higher in metastases than in the primary tumours (Mehnert et al, 2010). On the other hand, the anti-angiogenic VEGFA165b is downregulated in metastatic melanomas and seems to predict their metastatic spread (Pritchard-Jones et al, 2007). Here, we report that orlistat significantly reduces experimental metastases, clearly demonstrating a cytotoxic effect against melanoma cells. Moreover, this drug inhibits angiogenesis associated with B16-F10 tumours and modulates VEGFA production.
Materials and methods
Cell culture
B16-F10 mouse melanoma and SK-MEL-25 human melanoma cells (ATCC, Manassas, VA, USA) were maintained in RPMI (Invitrogen, Camarillo, CA, USA) with 10% FBS (Cultilab, Campinas, Brazil). Human oral squamous carcinoma cells (SCC-9, ATCC) were grown in DMEM/F-12 (Invitrogen) with 10% FBS and 400 ng ml−1 hydrocortisone. Endothelial cells from the rabbit aorta (RAEC, kindly provided by Dr Helena B Nader, UNIFESP, Brazil) were cultured in HAM-F12 (Invitrogen) containing 10% FBS. Human umbilical vein endothelial cells (HUVECs) were maintened in RPMI 10% FBS. Orlistat (Xenical, Roche, Basel, Switzerland), prepared as described by Knowles et al (2004), or cerulenin (Sigma-Aldrich, St Louis, MO, USA) were used to inhibit FASN.
In vivo studies
The animal experiments were performed according to the Animal Ethics Committee in Animal Research of UNICAMP. For the lung metastases assay, 8-week-old male C57BL6 mice (68) were inoculated at the tail vein with 2 × 105 B16-F10 cells suspended in 100 μl of PBS. After 24 h, the animals received daily intraperitoneal injections of orlistat or its vehicle (Kridel et al, 2004) for 19 days, when were killed and dissected. After fixation in Bouin solution, lung colonies were counted in a dissection microscope and embedded in paraffin for H&E staning. Angiogenesis was analysed in 6-week-old male C57BL6 mice (78) by injecting B16-F10 cells (106) intradermally at the ventral skin. Simultaneously, animals started to be treated as described above and were killed 10 days latter. The skin with the tumour was removed and photographed in a dissection microscope. The length and area of tumour-directed vessels were analysed with the Scion Image software (Scion Corporation, Frederick, MD, USA).
Cell proliferation, apoptosis, and viability
For the proliferation curves, RAEC cells were seeded in 24-well plates (8 × 103 per well) and after 24 h serum-starved for the same period of time. After that, complete medium plus FASN inhibitors or their vehicles was added, and cells from triplicate wells trypsinised and counted in a Neubauer chamber. Flow cytometry experiments were performed as described by Zecchin et al (2011). Cell viability was determined by plating RAEC (3 × 104) or HUVEC (8 × 104) cells in 6-well culture plates with 3 (4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (Sigma) according to the manufacturer’s instructions. All experiments were repeated at least three times independently.
Capillary-like assay
The formation of capillary-like structures by RAECs and HUVECs (3 × 104) was evaluated as described elsewhere (Pyriochou et al, 2007). The extension of vessel-like structures developed after 16 h in the presence of FASN inhibitors or conditioned media was assessed with the ImageJ software (Scion Corporation).
Conditioned media and ELISA
Conditioned media were obtained by plating 3 × 105 B16-F10, 6 × 105 SK-MEL-25 and SCC-9 cells in T-75 culture flasks. After 24 h, cells were washed with pre-warmed PBS and cultured in RPMI 10% FBS for 48 h. Medium was removed, centrifuged at 1100 × g for 3 min, and used to incubate endothelial cells (80% conditioned medium diluted with fresh complete medium) previously seeded in 6-well plates (3 × 104 RAECs and 8 × 104 HUVECs) and serum starved for 24 h. To verify the role of VEGF165b in endothelial proliferation, monoclonal antibodies (10 μg ml−1, clone 56-1, R&D Systems, Minneapolis, MN, USA) were added to the conditioned medium obtained from SK-MEL-25 cells. After trypsinisation (from triplicate wells), endothelial cells were counted in a Neubauer chamber after 24 and 48 h. The concentration of VEGFA was determined with VEGFA enzyme-linked immunosorbent assays (VEGF Quantikine ELISA and VEGF DuoSet ELISA, R&D Systems) following the manufacturer’s instructions and normalised by the number of cells after the treatments.
siRNA
siRNAs (Table 1) were synthesised, annealed, and purified by the manufacturer (Stealth RNAi, Invitrogen). B16-F10 cells were transfected as described earlier Carvalho et al (2008). SK-MEL-25 and SCC-9 cells were transfected with 50 nM of the siRNAs by using jetPRIME (2 μl ml−1, Polyplus Transfection, Illkirch, France). As negative controls, cells were transfected with equimolar concentration of a nonspecific control oligo (Stealth RNAi Negative Control Duplexes, Medium GC, Invitrogen). Fatty acid synthase knockdown was assessed by western blotting 48 h after transfections (Carvalho et al, 2008).
RNA isolation and quantitative RT–PCR
Total RNA was obtained with the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. cDNAs were synthesised from 2 μg of total RNA using the First-Strand cDNA Synthesis SuperScripT II RT (Invitrogen). Primer sequences and GenBank accession numbers are listed in Table 2. Quantitative RT–PCR was conducted using SYBR Green Real-time PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The reaction conditions were 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 57 °C for 1 min in a StepOne Plus Real-Time PCR System (Applied Biosystems). Relative gene expression was determined by the 2-ΔΔCT or standard curve methods in a SDS software version 2.0 (Applied Biosystems).
Results
Orlistat inhibits lung colonisation by B16-F10 cells and reduces blood vessels at the periphery of experimental melanomas
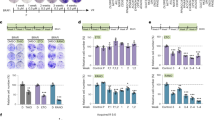
To evaluate the antimetastatic effects of orlistat in melanoma cells, we exploited the well-described lung colonisation assay, which represents a significant challenge for potential anticancer drugs. Twenty days after the inoculation of B16-F10 cells at the tail vein, the number of macroscopic lung colonies was decreased by 53.4% in orlistat-treated mice (Table 3 and Figures 1A–D). In addition, MMP-2 and -9 activities were not modified by the drug in cultured B16-F10 cells (data not shown). Ten days after the intradermal inoculation of B16-F10 cells, all mice showed well-developed tumours at the ventral skin, which were variable in size and shape. Both the length and area of peritumoral blood vessels were significantly reduced by the treatment with orlistat, in comparison with the controls (Figures 1E–J).
Orlistat inhibits lung colonisation by B16-F10 cells and peritumoral blood vessels in experimental melanomas. (A) Macroscopic aspects of the lungs from control mice, with many black B16-F10 colonies at the surface, which were clearly reduced in orlistat-treated animals (B). (C and D) Histological sections showing B16-F10 metastatic colonies at the periphery and central portion of the lung (arrows). Representative images from three independent experiments (original magnification: C: × 25 and D: × 100, H&E staining). (E–H) Representative images of the mouse ventral skin showing blood vessels at the periphery of the tumour. (E) Blood vessels directed to the tumour of a control animal (arrows) and (F) detail of the small peritumoral blood vessels of the same animal shown in E. (G) Animal treated with orlistat and (H) detail of G. Orlistat-treated mice showed a significant reduction in both length (I) and area (J) of peritumoral blood vessels in comparison with the controls treated with the vehicle ethanol. The total length of the more calibrous blood vessels (E and G) and the area of small blood vessels (represented by the dashed lines in F and H) were estimated with the aid of a computer programme. All measurements were normalised by the area of the tumour (C: ethanol control; orl: orlistat; *P=0.024; **P=0.002, Mann–Whitney test; original magnification: E and G: × 8, F and H: × 20).
Effects of FASN inhibitors on RAEC endothelial cells
The growth curves shown in Figures 2A and B show an important inhibitory effect of both cerulenin and orlistat on RAEC cell proliferation. Accordingly, these drugs promoted an increase of the G0–G1 and a clear reduction of the S phase, in comparison with the untreated controls (Figures 2C and D). In addition, cerulenin and orlistat reduced the viability of RAEC cells in a dose–response manner, enhanced apoptosis, and inhibited the formation of capillary-like structures in matrigel (Figures 2E–K).
FASN is essential for rabbit aortic endothelial cell (RAEC) growth and survival. (A and B) 0.75 μg ml−1 of cerulenin (A) or 100 μ M of orlistat (B) strongly reduced the proliferation of RAEC cells cultured in standard conditions (10% of FBS; -▴-), in comparison with the respective controls (-▪-; *P<0.001, Mann–Whitney test). (C and D) Cell cycle analysis by flow cytometry demonstrated that the incubation of RAEC cells with 0.75 μg ml−1 of cerulenin for 48 h or 100 μ M of orlistat for 96 h enhances the G0–G1 population and reduces the number of cells in the S phase (▪ G0/G1; □ S  ; G2/M; *P<0.05, t-test). (E and F) 3 (4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide experiments showing that the viability of RAEC cells is reduced by cerulenin or orlistat in a dose–response manner (*P<0.05, t-test). (G and H) The percentage of B16-F10 cells in apoptosis was significantly enhanced following the treatment with 0.75 μg ml−1 of cerulenin or 100 μ M of orlistat for 48 h (*P<0.05, t-test). (I–L) The length of capillary-like structures formed by RAEC cells in matrigel was significantly reduced by both drugs (C: control with dimethyl sulfoxide for cerulenin or ethanol for orlistat; cer: cerulenin; orl: orlistat; original magnification in K: × 40).
; G2/M; *P<0.05, t-test). (E and F) 3 (4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide experiments showing that the viability of RAEC cells is reduced by cerulenin or orlistat in a dose–response manner (*P<0.05, t-test). (G and H) The percentage of B16-F10 cells in apoptosis was significantly enhanced following the treatment with 0.75 μg ml−1 of cerulenin or 100 μ M of orlistat for 48 h (*P<0.05, t-test). (I–L) The length of capillary-like structures formed by RAEC cells in matrigel was significantly reduced by both drugs (C: control with dimethyl sulfoxide for cerulenin or ethanol for orlistat; cer: cerulenin; orl: orlistat; original magnification in K: × 40).
Effects of orlistat on VEGFA expression
Differences in the expression of total mouse VEGFA within the experimental melanoma tissues were not detected after the treatment with orlistat (data not shown). Therefore, we next investigated the expression of VEGFA in melanoma cell lines. Total VEGFA was upregulated in B16-F10 cells treated with cerulenin or orlistat (Figures 3A and B). Total human VEGFA, VEGFA121, 165, 189, and 165b were also increased by both drugs in SK-MEL-25 and SCC-9 cells (Figures 3C–F). Vascular endothelial growth factor A189b and 121b were not expressed by these cell lines.
FASN inhibitors enhance VEGFA expression. Quantitative RT–PCR analysis of total mouse VEGFA in B16-F10 cells (A and B) and total human VEGFA, VEGFA 189, 165, 121, and 165b transcripts in SK-MEL-25 (C and D) and SCC-9 cells (E and F) after 24 h of treatment with cerulenin (cer) or orlistat (orl). Fatty acid synthase inhibitors at different concentrations increased both total VEGFA and VEGFA isoforms, in comparison with the respective controls (C: control with dimethyl sulfoxide for cerulenin or ethanol for orlistat; expression levels in control cells=1; *P<0.05, t-test).
Conditioned media from orlistat-treated cancer cells do not stimulate or inhibit endothelial cell growth
To check whether VEGFA(s) released by B16-F10 cells stimulate the proliferation of RAEC endothelial cells, we first estimated their concentration in the cell culture medium. As expected, B16-F10 cells produced more total VEGFA than RAEC cells, and the conditioned medium from the former stimulates the proliferation of the latter (Figures 4A and B). Similarly, conditioned media from SK-MEL-25 or SCC-9 cells increase the growth rate of HUVEC cells (Figures 4C and D). Orlistat stimulates the secretion of total VEGFA, which was confirmed by the FASN knockdown with specific siRNAs in both B16-F10 and SK-MEL-25 cells (Figures 5A–C and E–G). The production of VEGFA165b by SK-MEL-25 cells was also significantly increased after the incubation with the drug (Figure 5H). Similar results were found in SCC-9 cells (data not shown). Next, we sought to verify whether the orlistat-induced VEGFA(s) are pro- or anti-angiogenic by incubating RAEC or HUVEC cells with culture medium previously conditioned by orlistat-treated cancer cells. As depicted in Figure 5D, RAEC cell growth was not changed by the cell culture medium from orlistat-treated B16-F10 cells, although cell death occurred after 48 h with the conditioned medium from control cells. Interestingly, conditioned media from orlistat-treated SK-MEL-25 (Figure 5I) or SCC-9 cells (data not shown) significantly inhibited the proliferation of HUVEC cells. To verify whether the endothelial cell growth inhibition induced by the treatment of cancer cells with orlistat was associated with VEGFA165b, the only inhibitory isoform detected, specific antibodies were used to neutralise its activity. Indeed, as depicted in Figure 5J, the proliferation of HUVECs was restored in conditioned medium from orlistat-treated SK-MEL-25 cells containing anti-VEGFA165b antibodies, strongly suggesting an anti-angiogenic role for the orlistat-induced secretion of VEGF165b by tumour cells.
(A) Total VEGFA protein levels in B16-F10-conditioned medium is higher than in RAEC-conditioned medium after 24 and 48 h. The growth of RAEC (B) and HUVEC (C and D) cells is stimulated by the incubation with culture medium previously conditioned by B16-F10 (CM-B16-F10), SK-MEL-25 (CM-SK-MEL-25), or SCC-9 (CM-SCC-9) cells, in contrast with medium conditioned by RAEC (CM-RAEC) or HUVEC (CM-HUVEC) cells (*P<0.05, t-test).
Total VEGFA production by B16-F10 and SK-MEL-25 melanoma cells is stimulated by the treatment with orlistat (orl) for 48 h (A and E) or FASN knockdown with specific siRNAs (C and G). Western blotting reactions confirming FASN knockdown in the siRNAs transfected B16-F10 (B) and SK-MEL-25 (F) cell lysates. (H) The incubation with 300 μ M of orlistat for 48 h enhances the secretion of VEGF165b by SK-MEL-25 cells. (D) Conditioned medium from orlistat-treated B16-F10 cells does not affect the growth of RAEC cells after 24 h. At 48 h, RAEC cells were well preserved in the presence of medium from orlistat-treated B16-F10 cells, while cell death was observed with control medium. (I) Conditioned medium from orlistat-treated SK-MEL-25 cells significantly decreased the growth of HUVEC cells after 48 h (*P<0.05, t-test). (J) The proliferation of HUVEC cells in conditioned medium from orlistat-treated SK-MEL-25 cells is restored in the presence of 10 μg ml−1 of neutralising anti-VEGFA165b monoclonal antibodies. C: ethanol or nonspecific siRNA oligos.
In contrast with RAEC cells (Figures 2E and F), HUVEC cell viability and length of the capillary-like structures formed in matrigel were not affected by cerulenin or orlistat (Figures 6A–D). However, conditioned media from orlistat-treated cancer cells significantly decrease the length of capillary-like structures developed by these cells (Figures 6E–H), further suggesting that this drug induces an anti-angiogenic phenotype.
Effect of orlistat on the formation of capillary-like structures by HUVEC cells. (A and B) 3 (4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide experiments showing that the viability of HUVEC cells is not affected by the presence of cerulenin (cer) or orlistat (orl). (C and D) Orlistat alone (300 μ M) does not change the formation of capillary-like structures by HUVECs in matrigel. However, conditioned media (CM) from orlistat-treated (300 μ M) SK-MEL-25 or SCC-9 cells significantly decrease the length of capillary-like structures, in comparison with the respective controls (E–H). C: ethanol control.
Discussion
The association between FASN expression and activity with tumour growth and metastasis has been clearly demonstrated in several types of human malignancies. In fact, tumour cells synthesise fatty acids de novo, despite nutritional supply (Ookhtens et al, 1984; Weiss et al, 1986).
Here, we show that FASN is critical for the proliferation and survival of RAEC cells, as orlistat or cerulenin significantly reduced their growth, promoted apoptosis, and impaired the formation of capillary-like structures in vitro. Curiously, the viability of HUVECs was not affected by FASN inhibitors, possibly due to their lower proliferation rates in comparison with the immortalised RAECs. Despite the fact that FASN pharmacological blockage or knockdown inhibits cell cycle progression and cause apoptosis in many cancer cell lines (Pizer et al, 1998; Li et al, 2001; Kridel et al, 2004; Zhou et al, 2007; Migita et al, 2009), the role of FASN in nonmalignant cells is still uncertain. Indeed, we observed that the growth of gingival fibroblasts in primary cultures is reduced by cerulenin (Almeida et al, 2005). Furthermore, Browne et al (2006) described that orlistat inhibits the proliferation and promotes apoptosis in VEGFA-stimulated HUVECs.
We previously demonstrated that orlistat reduces proliferation and promotes apoptosis in B16-F10 cells (Carvalho et al, 2008; Zecchin et al, 2011). Both orlistat- and cerulenin-induced apoptotic cell death in this cell line occur through the intrinsic pathway, as demonstraded by the cytochrome c release and caspase-9 and -3 activation, independent of p53 activation or mithocondrial permeability transition (Zecchin et al, 2011). Importantly, we found that orlistat reduces B16-F10 cell metastatic spread from the peritoneal cavity to the mediastinal lymph nodes in a mouse model for melanoma spontaneous metastasis (Carvalho et al, 2008). This drug also inhibits metastasis of oral squamous cell carcinoma to the cervical lymph nodes in an orthotopic mouse model (Agostini M, unpublished results). Here, we observed a remarkable reduction of lung colonies in the treated animals (50%), which demonstrates the cytotoxic effect of orlistat on B16-F10 cells and further suggests that FASN activity may be targeted in melanoma chemotherapy.
This study shows for the first time that FASN modulates tumour angiogenesis through differential expression of VEGFA isoforms. The biological mechanisms responsible for this phenomenon are unknown. However, preliminary results from our laboratory show that the in vitro proteasomal degradation of HIF-1α by B16-F10 protein lysates is accelerated by orlistat (Agostini M, unpublished results), suggesting that the downregulation of this transcription factor contributes for the anti-angiogenic phenotype. Vascular endothelial growth factor A, a potent growth factor for blood vessel endothelial cells, is also known to regulate vascular permeability (Dvorak et al, 1995) then contributing for the metastatic spread Karamysheva et al, 2008). The gene encoding human VEGFA is formed by eight exons (Tischer et al, 1991) from which seven isoforms are produced through alternative splicing: VEGFA121, 145, 148, 165, 189, 183, and 206 (Harper and Bates, 2008). Mouse transcripts contain one amino acid less in each isoform (Shima et al, 1996; Catena et al, 2007). An additional splice site in exon 8 generates the VEGFAxxxb family of anti-angiogenic peptides, that differ from their angiogenic counterparts in the last six residues (Bates et al, 2002; Woolard et al, 2004; Perrin et al, 2005; Cébe Suarez et al, 2006) and are downregulated in renal, colorectal, and prostate carcinomas (Bates et al, 2002; Díaz et al, 2008; Varey et al, 2008), metastatic melanomas (Pritchard-Jones et al, 2007), and diabetic retinopathies (Perrin et al, 2005). However, the anti-angiogenic activity of these factors is not completely characterised, as VEGFA121b and VEGFA165b were recently shown to be weakly angiogenic (Catena et al, 2010). In agreement with Menendez et al (2005b), which observed increased VEGFA in Her-2/Neu-overexpressing breast cancer cells following FASN inhibition with C75, we observed that orlistat and FASN knockdown raise the production of VEGFA(s) in B16-F10, SK-MEL-25, and SCC-9 cells. In this study, we found that VEGFA(s) produced by B16-F10 in the presence of orlistat do not increase the proliferation of RAEC endothelial cells. On the other hand, conditioned media from orlistat-treated human cancer cells (SK-MEL-25 and SCC-9) decreased the proliferation of HUVEC cells as well as the length of capillary-like structures in matrigel.
The expression of VEGFA120 in our mouse melanoma specimens (data not shown) is consistent with previous findings in human melanomas (Potgens et al, 1995; Redondo et al, 2000; Yu et al, 2002), in which VEGFA121 seems to be involved in peritumoral vascularisation due to its high diffusibility (Grunstein et al, 2000). This isoform was also associated with vasodilation and increased permeability of peritumoral vessels (Küsters et al, 2003). Our orlistat-treated melanomas also showed enhanced expression of VEGFA188 (data not shown). Vascular endothelial growth factor (VEGFA189), its human counterpart, when overexpressed in Mel57 melanoma cells promotes less pronounced vasodilatation than VEGFA165 and VEGFA121 (Küsters et al, 2003) and is nontumorigenic in WM1341B early-stage melanoma cells (Yu et al, 2002). Thus, as the complete organisation of the murine VEGFA gene is not still available, we searched for these factors in SK-MEL-25 human melanoma cells and found that FASN inhibitors significantly stimulate VEGFAs121, 165, 189, and 165b. Therefore, it is possible to hypothesise that overexpression of a particular sub-set of VEGFA isoforms have, at least in part, a role in the reduction of melanoma peritumoral angiogenesis that follows orlistat treatment. Importantly, the endothelial cell growth inhibiton promoted by human cancer cell lines was reversed by anti-VEGF165b neutralising antibodies, indicating a major role for this factor as an orlistat-induced gene product. In fact, VEGFA165b is downregulated in metastatic melanomas and seems to predict their metastatic spread (Pritchard-Jones et al, 2007).
In summary, here we show that FASN activity reduces both metastases and peritumoral angiogenesis in experimental melanomas. In addition, the reduction of endothelial cell growth and organisation of capillary-like structures in vitro further indicate a VEGFA165b-mediated anti-angiogenic effect of orlistat. Taken together, these observations suggest that FASN inhibition with orlistat may help to restrain melanoma metastatic dissemination.
Accession codes
Accessions
GenBank/EMBL/DDBJ
Change history
18 August 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP (2005) Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 24: 39–46
Almeida JP, Coletta RD, Silva SD, Agostini M, Vargas PA, Bozzo L, Graner E (2005) Proliferation of fibroblasts cultured from normal gingiva and hereditary gingival fibromatosis is dependent on fatty acid synthase activity. J Periodontol 76: 272–278
Alò PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE, Di Tondo U (2000) Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep 7: 1383–1388
Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62: 4123–4131
Browne CD, Hindmarsh EJ, Smith JW (2006) Inhibition of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. FASEB J 20: 2027–2035
Carvalho MA, Zecchin KG, Seguin F, Bastos DC, Agostini M, Rangel AL, Veiga SS, Raposo HF, Oliveira HC, Loda M, Coletta RD, Graner E (2008) Fatty acid synthase inhibition with Orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int J Cancer 123: 2557–2565
Catena R, Larzabal L, Larrayoz M, Molina E, Hermida J, Agorreta J, Montes R, Pio R, Montuenga LM, Calvo A (2010) VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Mol Cancer 9: 320–324
Catena R, Muniz-Medina V, Moralejo B, Javierre B, Best CJ, Emmert-Buck MR, Green JE, Baker CC, Calvo A (2007) Increased expression of VEGF121/VEGF165-189 ratio results in a significant enhancement of human prostate tumor angiogenesis. Int J. Cancer 120: 2096–2109
Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K (2006) A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci 63: 2067–2077
Díaz R, Peña C, Silva J, Lorenzo Y, García V, García JM, Sánchez A, Espinosa P, Yuste R, Bonilla F, Domínguez G (2008) p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer 123: 1060–1067
Dowling S, Cox J, Cenedella RJ (2009) Inhibition of fatty acid synthase by Orlistat accelerates gastric tumor cell apoptosis in culture and increases survival rates in gastric tumor bearing mice in vivo. Lipids 44: 489–498
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039
Einspahr JG, Thomas TL, Saboda K, Nickolof BJ, Warneke J, Curiel-Lewandrowski C, Ranger-Moore J, Duckett L, Bangert J, Fruehauf JP, Alberts DS (2007) Expression of vascular endothelial growth factor in early cutaneous melanocytic lesion progression. Cancer 110: 2519–2527
Erhard H, Rietveld FJ, van Altena MC, Bröcker EB, Ruiter DJ, de Waal RM (1997) Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res 7: S19–S26
Furuya Y, Akimoto S, Yasuda K, Ito H (1997) Apoptosis of androgen-independent prostate cell line induced by inhibition of fatty acid synthesis. Anticancer Res 17: 4589–4593
Gansler TS, Hardman W, Hunt DA, Schaffel S, Hennigar RA (1997) Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28: 686–692
Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS (2000) Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature. Mol Cell Biol 20: 7282–7291
Harper SJ, Bates DO (2008) VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8: 880–887
Innocenzi D, Alò PL, Balzani A, Sebastiani V, Silipo V, La Torre G, Ricciardi G, Bosman C, Calvieri S (2003) Fatty acid synthase expression in melanoma. J Cutan Pathol 30: 23–28
Kapur P, Rakheja D, Roy LC, Hoang MP (2005) atty acid synthase expression in cutaneous melanocytic neoplasms. Mod Pathol 18: 1107–1112
Karamysheva AF (2008) Mechanisms of angiogenesis. Biochemistry 73: 935–948
Knowles LM, Axelrod F, Browne CD, Smith JW (2004) A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J Biol Chem 405: 279–305
Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW (2004) Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res 64: 2070–2075
Kuhajda FP (2000) Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16: 202–208
Küsters B, de Waal RM, Wesseling P, Verrijp K, Maass C, Heerschap A, Barentsz JO, Sweep F, Ruiter DJ, Leenders WP (2003) Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res 63: 5408–5413
Li JN, Gorospe M, Chrest FJ, Kumaravel TS, Evans MK, Han WF, Pizer ES (2001) Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res 61: 1493–149
Mehnert JM, McCarthy MM, Jilaveanu L, Flaherty KT, Aziz S, Camp RL, Rimm DL, Kluger HM (2010) Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays. Hum Pathol 41: 375–384
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7: 763–777
Menendez JA, Vellon L, Lupu R (2005a) Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann Oncol 16: 1253–1267
Menendez JA, Vellon L, Oza BP, Lupu R (2005b) Does endogenous fatty acid metabolism allow cancer cells to sense hypoxia and mediate hypoxic vasodilatation? Characterization of a novel molecular connection between fatty acid synthase (FAS) and hypoxia-inducible factor-1alpha (HIF-1alpha)-related expression of vascular endothelial growth factor (VEGF) in cancer cells overexpressing her-2/neu oncogene. J Cell Biochem 94: 857–863
Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E, Fiore C, Xie W, Kung AL, Febbo PG, Subramanian A, Mucci L, Ma J, Signoretti S, Stampfer M, Hahn WC, Finn S, Loda M (2009) Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst 101: 519–532
Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS (2008) Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol 26: 5713–5720
Ookhtens M, Kannan R, Lyon I, Baker N (1984) Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol 247: 146–153
Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ (2005) Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 48: 2422–2427
Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF (1998) Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res 58: 4611–4615
Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP (1996a) Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res 56: 2745–2747
Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP (1996b) Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res 56: 1189–1193
Potgens AJ, Lubsen NH, van Altena MC, Schoenmakers JG, Ruiter DJ, de Waal RM (1995) Vascular permeability factor expression influences tumor angiogenesis in human melanoma lines xenografted to nude mice. Am J Pathol 146: 197–209
Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO (2007) Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer 97: 223–230
Pyriochou A, Tsigkos S, Vassilakopoulos T, Cottin T, Zhou Z, Gourzoulidou E, Roussos C, Waldmann H, Giannis A, Papapetropoulos A (2007) Anti-angiogenic properties of a sulindac analogue. Br J Pharmacol 8: 1207–1214
Redondo P, Bandrés E, Solano T, Okroujnov I, García-Foncillas J (2000) Vascular endothelial growth factor (VEGF) and melanoma. N-acetylcysteine downregulates VEGF production in vitro. Cytokine 12: 374–378
Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M (2003) Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res 1: 707–715
Rossi S, Ou W, Tang D, Bhattacharya N, Dei Tos AP, Fletcher JA, Loda M (2006) Ntestinal stromal tumours overexpress fatty acid synthase. J Pathol 209: 369–375
Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D'Amore PA (1996) The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem 271: 3877–3883
da Silva SD, Cunha IW, Nishimoto IN, Soares FA, Carraro DM, Kowalski LP, Graner E (2009) Clinicopathological significance of ubiquitin-specific protease 2a (USP2a), fatty acid synthase (FASN), and ErbB2 expression in oral squamous cell carcinomas. Oral Oncol 45: 134–139
Silva SD, Perez DE, Alves FA, Nishimoto IN, Pinto CA, Kowalski LP, Graner E (2008) ErbB2 and fatty acid synthase (FAS) expression in 102 squamous cell carcinomas of the tongue: correlation with clinical outcomes. Oral Oncol 44: 484–490
Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G (2002) Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer 98: 19–22
Takahiro T, Shinichi K, Toshimitsu S (2003) Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin Cancer Res 9: 2204–2212
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266: 11947–11954
Ueda SM, Yap KL, Davidson B, Tian Y, Murthy V, Wang TL, Visvanathan K, Kuhajda FP, Bristow RE, Zhang H, Shih IeM (2010) Expression of Fatty Acid Synthase Depends on NAC1 and Is Associated with Recurrent Ovarian Serous Carcinomas. J Oncol 10: 1–12
Van de Sande T, Roskams T, Lerut E, Joniau S, Van Poppel H, Verhoeven G, Swinnen JV (2005) High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol 206: 214–219
Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, Harper SJ, Bates DO (2008) VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 98: 1366–1379
Visca P, Sebastiani V, Botti C, Diodoro MG, Lasagni RP, Romagnoli F, Brenna A, De Joannon BC, Donnorso RP, Lombardi G, Alo PL (2004) Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res 24: 4169–4173
Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F, Goggins M (2009) Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev 18: 2380–2385
Weiss L, Hoffmann GE, Schreiber R, Andres H, Fuchs E, Körber E, Kolb HJ (1986) Fatty-acid biosynthesis in man, a pathway of minor importance. Purification, optimal assay conditions, and organ distribution of fatty-acid synthase. Biol Chem 367: 905–912
Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO (2004) VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 64: 7822–7835
Yu JL, Rak JW, Klement G, Kerbel RS (2002) Vascular endothelial growth factor isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res 62: 1838–1846
Zecchin KG, Rossato FA, Raposo HF, Melo DR, Alberici LC, Oliveira HC, Castilho RF, Coletta RD, Vercesi AE, Graner E (2011) Inhibition of fatty acid synthase in melanoma cells activates the intrinsic pathway of apoptosis. Lab Invest 91: 232–240
Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP (2007) Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res 67: 2964–2971
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant 2008/57471-7. FS, MAC, DCB, MA, and KGZ were supported by the FAPESP fellowships (2010/50946-0, 2007/58158-8, 2010/51090-1, 2008/55548-2, and 2007/54639-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Seguin, F., Carvalho, M., Bastos, D. et al. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br J Cancer 107, 977–987 (2012). https://doi.org/10.1038/bjc.2012.355
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.355
Keywords
This article is cited by
-
LncRNA RPARP-AS1 promotes the progression of osteosarcoma cells through regulating lipid metabolism
BMC Cancer (2024)
-
Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies
Oncogenesis (2023)
-
The dynamic behavior of lipid droplets in the pre-metastatic niche
Cell Death & Disease (2020)
-
Adipophilin expression in cutaneous malignant melanoma is associated with high proliferation and poor clinical prognosis
Laboratory Investigation (2020)
-
The antimetastatic activity of orlistat is accompanied by an antitumoral immune response in mouse melanoma
Cancer Chemotherapy and Pharmacology (2020)