Abstract
Aim:
Metrnl is a novel secreted protein, but its physiological roles remain elusive. In this study, we investigated the tissue expression pattern of Metrnl in humans and explored its possible physiological role in the tissues with most highly expressed levels.
Methods:
A human tissue microarray containing 19 types of tissues from 69 donors was used to examine the tissue expression pattern of Metrnl, and the expression pattern was further verified in fresh human and mouse tissues. Intestinal epithelial cell-specific Metrnl knockout mice were generated, which were used to analyze the physiological roles of Metrnl.
Results:
Metrnl was the most highly expressed in the human gastrointestinal tract, and was specifically expressed in the intestinal epithelium. Consistent with this, Metrnl mRNA was also most highly expressed in the mouse gastrointestinal tract among the 14 types of tissues tested. In the intestinal epithelial cell-specific Metrnl knockout mice, the Metrnl levels in the gut fluid were significantly reduced, whereas the Metrnl serum levels showed a trend towards a reduction, but this change was not statistically significant. This cell-specific deletion of Metrnl did not affect body weight, food intake, blood glucose, colon length and histology, intestinal permeability, mucus content or mucin 2 expression under physiological conditions, but statistically decreased the expression of antimicrobial peptides, such as regenerating islet-derived 3 gamma (Reg3g) and lactotransferrin.
Conclusion:
Metrnl is highly expressed in the intestinal epithelial cells of humans and mice, which mainly contributes to the local gut Metrnl levels and affects the serum Metrnl level to a lesser extent. Metrnl plays a role in maintaining gut antimicrobial peptides.
Similar content being viewed by others
Introduction
Metrnl, also known as Cometin1, Subfatin2, or Interleukin 393, was recently identified as a secreted protein containing 311 amino acids with a 45-amino-acid signal peptide. Until now, there have only been a few studies exploring the function of Metrnl1,4,5,6.
Metrnl has a similar function to its only homologous gene, Meteorin (Metrn), which is a novel neurotrophic factor7. It has been demonstrated that recombinant Metrnl protein can accelerate neurite outgrowth and neuroblast migration in vitro and display a neuroprotective effect in vivo1.
We identified Metrnl as a novel adipokine during a process to screen new adipokines, demonstrating that Metrnl is abundant in subcutaneous adipose tissue in both humans and mice2. Metrnl expression is dramatically increased during adipogenesis and in white fat during obesity. Through generation of adipose-specific Metrnl transgenic overexpression and knockout mice, we demonstrate that Metrnl can upregulate PPARγ; promote white adipocyte differentiation, expandability, and lipid metabolism; inhibit adipose inflammation; and ultimately antagonize obesity-induced insulin resistance6.
It has also been reported that Metrnl is upregulated in white adipose tissue upon acute cold exposure and in muscle after an acute bout of concurrent exercise. Administration of recombinant Metrnl protein or an adenovirus encoding the Metrnl gene promotes the transient browning of white adipose tissue through an eosinophil-dependent increase of IL-4 expression and alterative (M2) macrophage activation5, suggesting its role in immune-adipose interactions5. A very recent study also implies that Metrnl is involved in both innate and acquired immune responses because Metrnl expression is upregulated in some human inflammation- or immune-associated diseases3.
Due to limited studies on Metrnl function, we are far from clear regarding the roles of Metrnl in various tissues. Furthermore, the tissue expression pattern of Metrnl in humans has not been examined at the protein level, although the tissue expression of Metrnl has previously been detected at the RNA level in our and other studies2,3. In this study, we examined the tissue expression pattern of Metrnl using human tissue microarray and found that Metrnl is very highly expressed in epithelial cells of the gastrointestinal tract, especially in the colon epithelium. Furthermore, we generated intestinal epithelial cell-specific Metrnl knockout mice to investigate the basic phenotypes of Metrnl in intestinal epithelial cells.
Materials and methods
Human multi-organ tissue microarray
A commercial tissue microarray (OD-NH-Com01-003) was purchased from Shanghai Outdo Biotech Co, Ltd and was constructed from formalin-fixed, paraffin-embedded human samples. The tissue microarray was prepared by dot-arraying 19 different types of tissues originating from 69 donors. The details of the samples are listed in Table 1.
All research involving human subjects was approved by the medical ethical committee of the Second Military Medical University, and written informed consent was obtained from all subjects.
Immunohistochemical staining for Metrnl
Immunohistochemical staining of human and mouse tissues using Metrnl antibody (Sigma) was performed on slides according to the protocol we previously described2,6. Briefly, slides were incubated at 60 °C for 20 min. After routine deparaffinization and rehydration, the slides were pretreated with 10 mmol/L sodium citrate buffer (pH 6.0) and boiled for 10 min for antigen retrieval. The endogenous peroxidase was quenched by adding hydrogen peroxide (3% H2O2 in 100% ethanol) at room temperature for 15 min. After washing with phosphate buffered saline (PBS), the slides were blocked with 10% non-immune goat serum. Then, the slides were incubated overnight with primary Metrnl antibody (1:100 dilutions) and washed with Tris-buffered saline. Next, the slides were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:1000) for 15 min. Immunoreactive complexes were detected with 3,3′-diaminobenzidene (DAB, Sigma). Finally, the slides were counter-stained with hematoxylin before microscopic analysis.
Generation of intestinal epithelial cell-specific Metrnl knockout mice
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the animal ethical committee of the Second Military Medical University. Metrnlloxp/loxp mice were generated as we described elsewhere6. Villin-cre mice [B6.Cg-Tg(Vil1-cre)1000Gum/J; JAX stock 021504] were used to generate intestinal epithelial cell-specific Metrnl knockout (Metrnl−/−) mice. The breeding strategy for the Metrnl−/− mice is displayed in Figure 2A. Briefly, Metrnlloxp/loxp mice were crossed to Villin-cre mice to generate Metrnlloxp/wtVillin-cre mice, which were crossed to Metrnlloxp/loxp mice to generate Metrnlloxp/loxp Villin-cre mice (Metrnl−/− mice). Then, Metrnl−/− mice were mated with Metrnlloxp/loxp mice, and their offspring were genotyped by polymerase chain reaction (PCR) analysis of genomic DNA. The littermate Metrnlloxp/loxp mice were used as the corresponding wild-type controls.
Breeding and identification of Metrnlloxp/loxp Villin-cre mice (Metrnl−/−). (A) Breeding strategy for Metrnl conditional knockout mice. (B) Genotyping of Metrnl-floxed mice. The PCR product of 243 bp is amplified from the conditional floxed (loxp-flanked) Metrnl allele, and the PCR product of 131 bp is amplified from the wild-type Metrnl allele. (C) Genotyping of Cre-positive mice, including Metrnlloxp/wt Villin-cre and Metrnlloxp/loxp Villin-cre mice. (D) Metrnl mRNA expression in the tissues of Metrnl−/− and wild-type mice. WAT, white adipose tissue. BAT, brown adipose tissue. n=6. The data are expressed as the mean±SEM. **P<0.01. (E) Immunohistochemical staining for Metrnl in the colons of Metrnl−/− and wild-type mice. Bar, 100 μm.
Detection of food intake and blood glucose
For the detection of food intake, the mice were placed in pairs in cages, and their food intake was monitored for 3 d. For the detection of blood glucose, mouse tail blood was used to measure the blood glucose levels at the basal state and at 2, 4, 6, 8, 10, and 12 h after fasting with OneTouch Glucose Meters (LifeScan), as described elsewhere8.
Fluorescein isothiocyanate-dextran assay
Wild-type and Metrnl−/− mice were fasted for 4 h and then administered 10 mg of fluorescein isothiocyanate (FITC)–dextran (molecular weight, 3000–5000 Da or 40000 Da; Sigma) by gavage as described elsewhere9. After 4 h, the mice were euthanized by cervical dislocation and bled by cardiac puncture. The blood was centrifuged at 3000×g for 30 min, and the serum was collected for fluorometry at Ex/Em 485/528 nm to detect the fluorescence intensity.
Quantification of mRNA by real-time PCR
Total RNA was extracted from various tissues using TRIzol reagent (Invitrogen), and real-time PCR was performed using an ABI 7500 Real-Time PCR System (Applied Biosystems) as previously described2. A final 20 μL reaction mixture included 10 μL SYBR Green, 2 μL cDNA template and 1 μL primers. The average threshold cycle (Ct) was determined from duplicate reactions, the target gene expression was normalized to GAPDH, and quantitative measurements were obtained using the ΔΔCT method. All primers are listed with their sequences in Table 2.
Histological staining
Periodic acid–Schiff (PAS) and hematoxylin and eosin (H&E) staining were performed as previously described10,11. Briefly, the intestine samples used for histological analysis were fixed with 4% phosphate buffered paraformaldehyde for 24 h and embedded in paraffin. Sections of 4-μm thickness were prepared and stained with PAS or HE. Images were captured using a Leica microscope. Semiquantitative analysis of PAS staining was performed using ImageJ software.
Gut fluid collection
The method for intestinal fluid collection followed a previously described procedure12. Briefly, after fasting overnight, mice were euthanized with an overdose of pentobarbital sodium13, and the abdomen was immediately opened to excise identical 5 cm lengths of colon. First, the colon samples were incubated with 8 mL PBS for 10 min. Second, a syringe with a blunt needle was used to flush PBS three times from the proximal to distal ends of the lumen of the colon. Then, the PBS was collected and centrifuged at 12 000×g at 4 °C for 10 min. The supernatant was collected and filtered using Amicon® Ultra-4 Centrifugal Filters (molecular weight cutoff 10 kDa, Merck). The concentrated liquid was slightly adjusted to the same volume with PBS. Finally, the Metrnl concentration was determined using the DuoSet Development kit (R&D Systems).
Statistical analysis
All data are presented as the mean±SEM and were analyzed using Prism 5.0 software (GraphPad Software). Statistical significance was determined by a two-tailed Student's t-test. P<0.05 was considered to be statistically significant.
Results
High expression of Metrnl in the human gastrointestinal tract
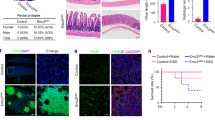
To investigate the expression pattern of Metrnl in different human tissues, we performed multi-organ human tissue microarray. Most of the tissues evaluated comprised four samples from different donors with different ages. According to the results of immunohistochemical staining, the tissues could be divided into 4 grades: negative, weak positive, positive, and strong positive. As shown in Table 3, we did not detect Metrnl expression in the brain, cerebellum, skeletal muscle, ovary, prostate, heart, pancreas or uterus, which was in accordance with our previous study2. The expression of Metrnl in the liver and adrenal glands was weakly positive. Metrnl was expressed in the kidney, thyroid, testis, spleen and lung. Notably, Metrnl expression in the gastrointestinal tract and skin was strongly positive and dramatically higher than that in other tissues (Table 3, Figure 1A).
High expression of Metrnl in the gastrointestinal tracts of humans and mice. (A) Immunohistochemical staining of representative human tissues. Strong positive immunostaining in the colon and small intestine, moderate positive immunostaining in the thyroid and kidney, weak positive immunostaining in the liver and adrenal glands, and nearly negative immunostaining in the brain and skeletal muscle were observed. Bar, 50 μm. Staining of Metrnl is indicated by a yellow arrow. (B) Metrnl expression in human intestinal epithelial cells. Bar, 200 μm. (C) Metrnl mRNA expression in 14 tissues from C57BL/6 mice, with the highest expression in the colon, followed by the small intestine and stomach.
To determine in which types of cells Metrnl expression occurred, more fresh human intestine tissues were collected, and Metrnl expression was detected with immunohistochemistry. The results showed that Metrnl was mainly expressed in the epithelial cells of the intestine but not in other cell types (Figure 1B).
To further identify whether Metrnl displays a similar expression pattern in the mouse, we detected Metrnl expression in different mouse tissues with real-time PCR. Consistently, Metrnl expression was very high in the mouse gastrointestinal tract, especially in the colon. Metrnl expression in the adipose tissue, lung, testis and kidney was relatively high, whereas its expression in the brain, spleen or pancreas was much lower (Figure 1C).
Deficiency of Metrnl in intestinal epithelial cells does not cause visible differences
To understand the role of Metrnl in intestinal epithelial cells, we generated intestinal epithelial cell-specific Metrnl knockout mice by mating Metrnlloxp/loxp mice and Villin-cre mice (Figure 2A–2C). The knockout efficiency of Metrnl was evaluated by both real-time PCR and immunohistochemistry. Metrnl expression was reduced by 96% in the colon and showed no changes in the heart, liver, muscle and adipose tissue (Figure 2D). Consistent with this, immunohistochemical staining demonstrated that Metrnl protein expression in the colon epithelial cells of Metrnl−/− mice was much lower than that in wild-type mice (Figure 2E). These results confirmed that Metrnl in intestinal epithelial cells was specifically knocked out in Metrnl−/− mice.
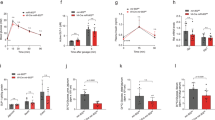
The ratio of Metrnl−/− and Metrnlloxp/loxp mice followed Mendel's law, and no visible differences were observed between the Metrnl−/− and wild-type mice. The body weight and food intake of Metrnl−/− mice showed no difference from wild-type mice (Figure 3A and 3B). The blood glucose levels were comparable between Metrnl−/− and wild-type mice under both fed and fasting conditions (Figure 3C).
Effects of intestinal epithelial Metrnl on body weight, food intake and blood glucose. (A) The body weight of Metrnl−/− mice is comparable to that of wild-type mice fed a normal chow diet. (B) Food intake of Metrnl−/− and wild-type mice fed a normal chow diet. n=12. (C) Blood glucose levels were measured at the indicated time points after fasting in Metrnl−/− and wild-type mice fed a normal chow diet. n=7. Mean±SEM.
We further measured the length of the large intestine, which was unchanged in Metrn1−/− mice (Figure 4A). We also did not observe histological differences between the intestines of Metrnl−/− and wild-type mice using H&E staining (Figure 4B). In addition, to test the influence of Metrnl on intestinal permeability, the fluorescence intensity of FITC in the serum was examined after gavage with FITC-conjugated dextran at different molecular weights. As shown in Figure 4C, no difference was found between Metrnl−/− and wild-type mice in either the small-molecular-weight dextran group or the high-molecular-weight dextran group.
Effects of intestinal epithelial Metrnl on colon length, intestinal permeability and histology, serum Metrnl levels, and the level of Metrnl in the gut fluid. (A) Metrnl−/− mice display no difference in colon length compared with wild-type mice. (B) H&E staining of the colon in Metrnl−/− and wild-type mice. Bar, 200 μm. (C) The fluorescence intensity of FITC in the serum is comparable between Metrnl−/− and wild-type mice after gavage with FITC-conjugated dextran at the indicated molecular weight. (D) The serum concentration of Metrnl tended towards a decrease in Metrnl−/− mice compared to wild-type mice. n=7. (E) The Metrnl level in the gut fluid is significantly reduced in Metrnl−/− mice. n=7. The data are expressed as the mean±SEM. *P<0.05.
Deficiency of Metrnl in intestinal epithelial cells reduces the Metrnl level in the gut fluid
Considering that the Metrnl protein existed in both the vesicles and cytoplasm of epithelial cells, it could be secreted into the blood and be exported into the gut fluid via exocytosis. To determine whether intestine epithelial cells are the major source of circulating Metrnl, we compared the serum concentration of Metrnl between Metrnl−/− and wild-type mice using an ELISA kit. As shown in Figure 4D, the Metrnl serum level tended towards being reduced in Metrnl−/− mice but the difference was not significant. However, the Metrnl concentration in the gut fluid was significantly reduced in Metrnl−/− mice (Figure 4E).
Deficiency of Metrnl in intestinal epithelial cells does not change mucin production but downregulates antimicrobial peptides
One of the major functions of epithelial cells is the secretion of mucus14,15,16,17. To clarify whether Metrnl in epithelial cells regulates mucin expression, periodic acid-Schiff staining was performed, which showed that the mucus content in epithelial cells was not changed in Metrnl−/− mice (Figure 5A). Mucin 2 is particularly prominent in the gut and is secreted by epithelial cells18,19,20,21. Hence, we further detected mucin 2 expression with real-time PCR, finding that its expression was also unchanged (Figure 5B).
Effects of intestinal epithelial Metrnl on mucus production and antimicrobial gene expression. (A) Periodic acid-Schiff staining of the colon in Metrnl−/− and wild-type mice. Semiquantitative analysis was performed using ImageJ software. n=5. Bar, 200 μm. (B) Relative expression of mucin 2 and antimicrobial genes in the colons of Metrnl−/− and wild-type mice. n=6. The data are expressed as the mean±SEM. *P<0.05.
Intestinal epithelial cells, especially Paneth cells, produce a large number of antimicrobial peptides to prevent microorganism translocation and modulate microbial populations at the mucosal surface22,23. Thus, we further evaluated the expression of antimicrobial peptides in the intestine. As shown in Figure 5B, contrary to the lack of change in Villin expression in Metrnl−/− mice, the expression of most antimicrobial peptides, such as regenerating islet-derived 3 gamma (Reg3g)24,25,26 and lactotransferrin27, was significantly decreased. Others, such as serum amyloid A-3 (SAA3)28 and regenerating islet-derived 3 beta (Reg3b)25,29, showed a trend towards a decrease. This suggests that Metrnl plays a role in regulating the expression of antimicrobial peptides.
Discussion
In the present study, we demonstrated the expression pattern of Metrnl in various tissues, especially in human tissues. We found that Metrnl was abundantly expressed in both human and mouse gastrointestinal tracts. Immunohistochemistry results further demonstrated that Metrnl was mainly expressed in intestinal epithelial cells. To explore its function in intestinal epithelial cells, we generated intestinal epithelial cell-specific Metrnl knockout mice. No visible difference was observed between Metrnl−/− and wild-type mice. The body weight, food intake, blood glucose, length of the intestine, intestinal permeability and mucus secretion remained unchanged in Metrnl−/− mice. The Metrnl serum level showed a tendency towards a decrease, but no significant difference was observed between Metrnl−/− mice and wild-type mice. However, we indeed detected a significant reduction of the Metrnl level in the gut fluid and a marked downregulation of antimicrobial peptides in the intestinal tissue of Metrnl−/− mice.
During the process of measuring the tissue expression pattern of Metrnl, we found that Metrnl was extremely highly expressed in both the human and the mouse gastrointestinal tract. Using an immunohistochemistry method, we further identified that Metrnl was exclusively expressed in the epithelial cells of the intestine. Consistent with our finding, a previous study reported that Metrnl was highly expressed in barrier tissues3. In accordance with this, we also found that Metrnl expression was abundant in the lung and skin. Conclusively, this study, combined with the previous reports from our and other labs1,2,3,6, demonstrates that Metrnl expression is the highest in the gastrointestinal tract and skin, relatively high in white adipose tissue and the lung, and much lower in the muscles, spleen, pancreas and brain.
Intestinal epithelial cells are uniquely located at the border of luminal commensal microbiota and the lamina propria of the intestine30,31. Secretory intestinal epithelial cells can secrete mucins and antimicrobial proteins to establish a physical and biochemical barrier between the bacterial and epithelial surfaces22,30. To explore the function of Metrnl in intestinal epithelial cells, we generated intestinal epithelial cell-specific Metrnl knockout mice. Body weight, food intake and blood glucose were not changed in these mice, suggesting that Metrnl may not significantly influence intestinal nutrient absorption under physiological conditions. The length, permeability and histology of the intestine were also not altered in Metrnl knockout mice, suggesting that Metrnl is unable to influence intestinal development and structure. Mucus content and mucin 2 expression in epithelial cells and the colon were also similar between Metrnl knockout and wild-type mice. Hence, Metrnl does not affect intestinal mucus secretion. However, our study observed that the ablation of Metrnl dramatically reduced the expression of several antimicrobial peptides, such as Reg3g and lactotransferrin, suggesting that Metrnl can regulate the production of antimicrobial peptides and may have a role in interactions between hosts and microorganisms. Because Metrnl is a secreted protein, it is unclear whether Metrnl changes antimicrobial peptide expression in a direct or indirect way. Thus, the specific mechanism still needs to be further explored.
Considering that Metrnl expression was the highest in the intestinal epithelium among the evaluated tissues, we wondered whether it is the major source of blood Metrnl in systemic circulation. The Metrnl serum level was not statistically and significantly decreased in Metrnl−/− mice compared with wild-type mice, which does not support the notion that Metrnl released from intestinal epithelial cells is the main source of blood Metrnl. Correspondingly, the Metrnl level in the gut fluid was significantly decreased, indicating that Metrnl expressed in intestinal epithelial cells was mostly exported into the gut fluid. These results are consistent with the results of Metrnl immunohistochemistry in the intestine showing that Metrnl seems to exist mainly in exocytic vesicles (Figure 2E).
The function of Metrnl in intestinal epithelial cells or other mucous glands needs to be explored further. In this study, we showed that Metrnl regulated the expression of antimicrobial peptides. It has also been reported that Metrnl is upregulated in inflammatory disease and regulates immune cell aggregation and inflammatory factor release3,5. All these results, combined with the regulation of the local intestinal Metrnl level, suggest that Metrnl may participate in homeostasis of mucosal immunity and the development of inflammatory bowel disease, which need to be explored further.
In conclusion, we show that Metrnl is highly expressed in the intestinal epithelial cells of humans and mice. Metrnl can be secreted from intestinal epithelial cells, which mainly contributes to the local level of Metrn1 in the gut, and has less of an effect on the systemic circulating level of Metrnl. Metrnl seems not to play a critical role in intestinal development, nutrient absorption, or mucus secretion. However, Metrnl can significantly regulate the intestinal production of antimicrobial proteins.
Author contribution
Chao-yu MIAO designed the study and revised the manuscript; Zhi-yong LI and Mao-bing FAN wrote the manuscript; Zhi-yong LI, Mao-bing FAN, Sai-long ZHANG, Yi QU, Si-li ZHENG, and Jie SONG performed the experiments.
References
Jorgensen JR, Fransson A, Fjord-Larsen L, Thompson LH, Houchins JP, Andrade N, et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp Neurol 2012; 233: 172–81.
Li ZY, Zheng SL, Wang P, Xu TY, Guan YF, Zhang YJ, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther 2014; 20: 344–54.
Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol 2015; 156: 119–27.
Zheng SL, Li ZY, Song J, Liu JM, Miao CY . Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin 2016; 37: 571–9.
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157: 1279–91.
Li ZY, Song J, Zheng SL, Fan MB, Guan YF, Qu Y, et al. Adipocyte Metrnl antagonizes insulin resistance through PPARgamma signaling. Diabetes 2015; 64: 4011–22.
Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, Hamada H . Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J 2004; 23: 1998–2008.
Gan L, Meng ZJ, Xiong RB, Guo JQ, Lu XC, Zheng ZW, et al. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol Sin 2015; 36: 597–605.
Murchie R, Guo CH, Persaud A, Muise A, Rotin D . Protein tyrosine phosphatase sigma targets apical junction complex proteins in the intestine and regulates epithelial permeability. Proc Natl Acad Sci U S A 2014; 111: 693–8.
Jin J, Peng C, Wu SZ, Chen HM, Zhang BF . Blocking VEGF/Caveolin-1 signaling contributes to renal protection of fasudil in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2015; 36: 831–40.
Hu G, Liu J, Zhen YZ, Xu R, Qiao Y, Wei J, et al. Rhein lysinate increases the median survival time of SAMP10 mice: protective role in the kidney. Acta Pharmacol Sin 2013; 34: 515–21.
Bade S, Gorris HH, Koelling S, Olivier V, Reuter F, Zabel P, et al. Quantitation of major protein constituents of murine intestinal fluid. Anal Biochem 2010; 406: 157–65.
Xu TY, Lan XH, Guan YF, Zhang SL, Wang X, Miao CY . Chronic nicotine treatment enhances vascular smooth muscle relaxation in rats. Acta Pharmacol Sin 2015; 36: 429–39.
Hansson GC . Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 2012; 15: 57–62.
Johansson ME, Sjovall H, Hansson GC . The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 2013; 10: 352–61.
Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC . New developments in goblet cell mucus secretion and function. Mucosal Immunol 2015; 8: 712–9.
Kim YS, Ho SB . Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010; 12: 319–30.
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC . The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008; 105: 15064–9.
Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006; 131: 117–29.
Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295: 1726–9.
Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 2010; 6: e1000902.
Bevins CL, Salzman NH . Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9: 356–68.
Gallo RL, Hooper LV . Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012; 12: 503–16.
Cash HL, Whitham CV, Behrendt CL, Hooper LV . Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006; 313: 1126–30.
Burger-van Paassen N, Loonen LM, Witte-Bouma J, Korteland-van Male AM, de Bruijn AC, van der Sluis M, et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3beta, Reg3gamma and angiogenin-4. PLoS One 2012; 7: e38798.
Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG . MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 2007; 204: 1891–900.
Siqueiros-Cendon T, Arevalo-Gallegos S, Iglesias-Figueroa BF, Garcia-Montoya IA, Salazar-Martinez J, Rascon-Cruz Q . Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 2014; 35: 557–66.
Reigstad CS, Backhed F . Microbial regulation of SAA3 expression in mouse colon and adipose tissue. Gut Microbes 2010; 1: 55–7.
Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV . Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A 2008; 105: 20858–63.
Peterson LW, Artis D . Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14: 141–53.
Wittkopf N, Neurath MF, Becker C . Immune-epithelial crosstalk at the intestinal surface. J Gastroenterol 2014; 49: 375–87.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No 81130061, 81202572, and 81373414) and and Shanghai Science and Technology Innovation Action Plan (No 16JC1405100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Zy., Fan, Mb., Zhang, Sl. et al. Intestinal Metrnl released into the gut lumen acts as a local regulator for gut antimicrobial peptides. Acta Pharmacol Sin 37, 1458–1466 (2016). https://doi.org/10.1038/aps.2016.70
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.70
Keywords
This article is cited by
-
Chronic vascular pathogenesis results in the reduced serum Metrnl levels in ischemic stroke patients
Acta Pharmacologica Sinica (2024)
-
Metrnl regulates cognitive dysfunction and hippocampal BDNF levels in D-galactose-induced aging mice
Acta Pharmacologica Sinica (2023)
-
Metrnl deficiency retards skin wound healing in mice by inhibiting AKT/eNOS signaling and angiogenesis
Acta Pharmacologica Sinica (2023)
-
N-Acetylcysteine may exert hepatoprotective effect by regulating Meteorin-Like levels in Adriamycin-induced liver injury
Cell Stress and Chaperones (2023)
-
Roux-en-Y gastric bypass potentially improved intestinal permeability by regulating gut innate immunity in diet-induced obese mice
Scientific Reports (2021)