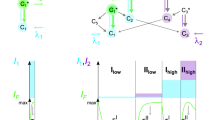
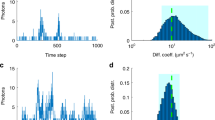
Abstract
Recent advances in fluorescence resonance energy transfer have led to qualitative and quantitative improvements in the technique, including increased spatial resolution, distance range, and sensitivity. These advances, due largely to new fluorescent dyes, but also to new optical methods and instrumentation, have opened up new biological applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Forster, T. Discuss. Faraday Soc. 27, 7–17 (1959).
Stryer, L. & Haugland, R.P. Proc. Natl. Acad. Sci., USA 58, 719–726 (1967).
Tsien, R.Y. Annu. Rev. Biochem. 67, 509–544 (1998).
Suzuki, Y., Yasunaga, T., Ohkura, R., Wakabayashi, T. & Sutoh, K. Nature 396, 380–383 (1998).
Tsien, R.Y. & Miyawaki, A. Science 280, 1954–1955 (1998).
Creemers, T.M., Lock, A.J., Subramaniam, V., Jovin, T.M. & Volker, S. Proc. Natl. Acad. Sci. USA 97, 2974–2978 (2000).
Matz, M.V., et al. Nature Biotech. 17, 969–973 (1999).
Wildt, S. & Deuschle, U. Nature Biotech. 17, 1175–1178 (1999).
Rice, S., et al. Nature 402, 778–784 (1999).
Glauner, K.S., Mannuzzu, L.M., Gandhi, C.S. & Isacoff, E.Y. Nature 402, 813–817 (1999).
Cha, A., Snyder, G.E., Selvin, P.R. & Bezanilla, F. Nature 402, 809–813 (1999).
Matulef, K., Flynn, G.E. & Zagotta, W.N. Neuron 24, 443–452 (1999).
Hu, Y.K. & Kaplan, J.H. J. Biol. Chem. 275, 19185–19191 (2000).
Callaci, S., Heyduk, E. & Heyduk, T. Mol Cell 3, 229–238 (1999).
Leonard, D.A. & Kerppola, T.K. Nature Struct. Biol. 5, 877–881 (1998).
Zlokarnik, G., et al. Science 279, 84–88 (1998).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Science 281, 269–272 (1998).
Schobel, U., Egelhaaf, H.-J., Brecht, A., Oelkrug, D. & Gauglitz, G. Bioconjugate Chem. 10, 1107–1114 (1999).
Ha, T., et al. Proc. Natl. Acad. Sci. USA 93, 624–628 (1996).
Sako, Y., Minoghchi, S. & Yanagida, T. Nature Cell Biol. 2, 168–172 (2000).
Weiss, S. Nature Struct. Biol. 7, 724–729 (2000).
Weiss, S. Science 283, 1676–1683 (1999).
Gruber, H.J., et al. Bioconjug Chem 11, 161–166 (2000).
Selvin, P.R. & Hearst, J.E. Proc. Natl. Acad. Sci. USA 91, 10024–10028 (1994).
Mathis, G. Clinical Chem. 41, 1391–1397 (1995).
Heyduk, E., Heyduk, T., Claus, P. & Wisniewski, J.R. J. Biol. Chem. 272, 19763–19770 (1997).
Root, D.D. Proc. Natl. Acad. Sci. USA 94, 5685–5690 (1997).
Xiao, M., et al. Proc. Natl. Acad. Sci. USA 95, 15309–15314 (1998).
Deniz, A.A., et al. Proc. Natl. Acad. Sci. USA 96, 3670–5 (1999).
Gadella, T.W.J., Jovin, T.M. & Clegg, R.M. Biophys. Chem. 48, 221–239 (1993).
Gadella, T.W.J. & Jovin, T.M. J. Cell Biol. 129, 1543–1558 (1995).
Ng, T., et al. Science 283, 2085–2089 (1999).
Siegel, R.M., et al. Science 288, 2354–2357 (2000).
Clegg, R.M. Methods Enzymol. 211, 353–388 (1992). (Note typographical error in equation 13.)
Murchie, A.I., et al. Nature 341, 763–766 (1989).
Clegg, R.M., Murchie, A.I., Zechel, A. & Lilley, D.M. Proc. Natl. Acad. Sci. USA 90, 2994–2998 (1993).
Jares-Erijman, E.A. & Jovin, T.M. J. Mol. Biol. 257, 597–617 (1996).
Tuschl, T., Gohlke, C., Jovin, T.M., Westhof, E. & Eckstein, F. Science 266, 785–789 (1994).
Acknowledgements
This work was supported by the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvin, P. The renaissance of fluorescence resonance energy transfer. Nat Struct Mol Biol 7, 730–734 (2000). https://doi.org/10.1038/78948
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78948
This article is cited by
-
A dual-mode nanoprobe based on silicon nanoparticles and Fe(II)-phenanthroline for the colorimetric and fluorescence determination of nitrite
Microchimica Acta (2023)
-
A Review on Forced Degradation Strategies to Establish the Stability of Therapeutic Peptide Formulations
International Journal of Peptide Research and Therapeutics (2023)
-
Quantitative Investigation of the Morphologically Corrugated CVD-Grown Graphene Monolayer Surface with a Nanoparticle-on-Mirror System
Plasmonics (2022)
-
Bioinspired Self-assembly Nanochaperone Inhibits Tau-Derived PHF6 Peptide Aggregation in Alzheimer’s Disease
Chinese Journal of Polymer Science (2022)