Abstract
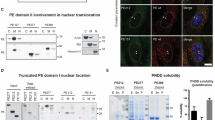
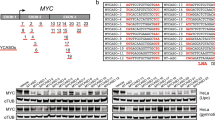
Peptide nucleic acids (PNA) are synthetic homologs of nucleic acids in which the phosphate-sugar polynucleotide backbone is replaced by a flexible polyamide. In this study, a PNA construct was employed as an anti-gene agent in intact cells in culture. The cell lines studied were derived from Burkitt's lymphomas (BL) that presented a translocated and hyperexpressed c-myc oncogene. A 17-mer anti-myc PNA, complementary to a unique sequence located at the beginning of the second exon of the oncogene, and was covalently linked at its N terminus to a nuclear localization signal (NLS) (PNA-mycwt-NLS). When BL cells were exposed to PNA-mycwt-NLS, the anti-gene construct was localized predominantly in the cell nuclei and a rapid consequent downregulation of c-myc expression occurred. Under these conditions, both completion of a productive cell cycle and apoptosis were inhibited.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Egholm, M., Buchardt, O., Nielsen, P.E. & Berg, R.H. Peptide nucleic acids (PNA). Oligonucleotide analogues with an achiral peptide backbone. J. Am. Chem. Soc. 114, 1895–1897 (1992).
Egholm, M. et al. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature 365, 566–568 (1993).
Demidov, V.V. et al. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem. Pharmacol. 48, 1310–1313 (1994).
Boffa, L.C., Morris, P.L., Carpaneto, E.M., Louissant, M. & Allfrey, V.G. Invasion of the CAG triplet repeats by a complementary Peptide Nucleic Acids inhibits transcription of the Androgen Receptor and TATA-Binding Protein genes and correlates with refolding of an active nucleosome containing a unique AR gene sequence. J. Biol. Chem. 271, 13228–13233 (1996).
Boffa, L.C., Carpaneto, E.M., Mariani, M.R., Louissant, M. & Allfrey, V.G. Contrasting effects of PNA invasion on chimeric DMMYC gene on transcription of its myc and PVT domains. Oncology Res. 9, 41–51 (1997).
Hanvey, C.J. et al. Antisense and antigene properties of Peptide Nucleic Acids. Science 258, 1481–1485 (1992).
Gray, D.G, Basu, S. & Wickstrom E. Transformed and immortalized cellular uptake of oligodeoxynucleoside phosphorothioates, 3′-alkylamino oligodeoxynucleotides, 2′-O-methyl oligoribonucleotides, oligodeoxynucleosides methylphosphonates, and Peptides Nucleic Acids. Biochem. Pharmacol. 53, 1465–1476 (1997).
Magrath, I. The pathogenesis of Burkitt's lymphoma. Adv. Cancer Res. 55, 133–270 (1990).
Cutrona, G., Ulivi, M., Fais, F., Roncella, S. & Ferrarini, M. Transfection of the c-myc oncogene into normal Epstein–Barr virus-harboring B cells results in new phenotypic and functional features resembling those of Burkitt Lymphoma cells and normal centroblasts. J. Exp. Med. 181, 699–711 (1995).
Spencer, C.A. & Groudine, M. Control of c-myc regulation in normal and neoplastic cells. Adv. Cancer Res. 56, 1–48 (1991).
Kalderon, D., Roberts, B.L., Richardson, W.D. & Smith, E.A. Short amino acid sequence able to specify nuclear localization. Cell 39, 499–509 (1984).
Gorlich, D. & Mattaj, I.W. Nucleocytoplasmic transport. Science 271, 1513–1518 (1996).
Branden, L.J., Mohamed, A.J. & Edvard Smith, C.I. Apeptide nucleic acid–nuclear localization signal fusion that mediates nuclear transport of DNA. Nat. Biotechnol. 17, 784–787 (1999).
Grazin, C. et al. Nucleotide sequence of the human c-myc locus: provocative open reading frame within the first exon. EMBO J. 3, 383–387 (1984).
Gangamani, B.P., Kumar, V.A. & Ganesh, N.K. Spermine conjugated peptide nucleic acids (spPNA): UV and fluorescence studies of PNA–DNA hybrids with improved stability. Biochem. Biophys. Res. Commun. 240, 778–782 (1997).
Colledge, W.H., Richardson, W.D., Edge, M.D. & Smith, A.E. Extensive mutagenesis of the nuclear localization signal of simian virus 40 large T antigen. Mol. Cell. Biol. 6, 4136–4138 (1986).
Saphire A.C., Bark S.J. & Gerace, L. All four homochiral enantiomers of a nuclear localization sequence derived from c-myc serve as functional import signals J. Biol. Chem. 273, 29764–29769 (1998).
Maky, L.A. & Bresslawer, K.Y. Calculating thermodinamics data for transition of any molecularity from equilibrium melting curves. Biopolymers 26, 1601–1620 (1987)
Tomac, S. et al. Ionic effects on the stability and conformation of Peptide Nucleic Acid complexes. J. Am. Chem. Soc. 118, 5544–5552 (1996).
Bentin, T. & Nielsen, P.E. Enhanced peptide nucleic acid binding to supercoiled DNA: possible implications for DNA "breathing" dynamics. Biochemistry 35, 8863–8869 (1996).
Tyler, B.M. et al. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood barrier and specifically reduce gene expression. Proc. Natl. Acad. Sci. USA 96, 7053–7058 (1999).
Boffa, L.C., Carpaneto, E.M. & Allfrey, V.G. Isolation of active genes containing CAG repeats after DNA strand invasion by Peptide Nucleic Acid. Proc. Natl. Acad. Sci. USA 92, 1901–1905 (1995).
Hyrup, B. & Nielsen, P.E. Peptide Nucleic Acids (PNA): synthesis, properties and potential applications. Bioorg. Med. Chem. 4, 5–23 (1996).
Roncella, S. et al. Apoptosis of Burkitt's Lymphoma cells induced by specific interaction surface IgM with a self-antigen: implications for lymphomagenesis in acquired immunodeficiency syndrome. Blood 88, 599–608 (1996).
Hueber, A.O. et al. Requirement for the CD95 receptor–ligand pathway in c-myc-induced apoptosis. Science 278, 1205–1209 (1998).
Pooga, M. et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 16, 856–861 (1998).
Clemens, G.B., Klein, G. & Povery, S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int. J. Cancer. 16, 125–133 (1975).
Abe, M., Nozawa, Y., Wakasa, H., Ohno, H. & Fukuhara, S. Characterization and comparison of two newly established Epstein–Barr virus-negative lymphoma B-cell lines. Cancer 61, 483–490 (1988).
Curtis Bird, R., Su, S. & Wu, G. in Cell biology : a laboratory handbook. (ed. Celis, J.E.) 278–281 (Academic Press, San Diego, CA; 1994).
Freid, J., Perez, A.G. & Clarkson, B.D. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures. J. Histochem. Cytochem. 26, 921–933 (1978).
Ausubel, F.M. et al. in Current protocols in molecular biology (eds. Ausubel, F.M. et al.) 1, 492–498 (John Wiley & Sons, Boston, MA, 1994).
Acknowledgements
We thank Prof. A. Alagòn (Instituto de Biotecnologia, UNAM, Cuernavaca, MX) and Prof. E. Melloni (Department of Experimental Medicine, Biochemistry Section, University of Genoa, Italy) for permission to use their microscopy facilities; X. Alvarado, A. Olvera, and Dr. M. Passalacqua for performing the confocal microscopy; and Dr. R. Stock for assistance in image analysis. This work was supported by: Associazione Italiana per la Ricerca sul Cancro (AIRC) and by CNR/CONACYT to L.C.B.; Istituto Superiore di Sanità (ISS) AIDS Project and AIRC to M.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cutrona, G., Carpaneto, E., Ulivi, M. et al. Effects in live cells of a c-myc anti-gene PNA linked to a nuclear localization signal. Nat Biotechnol 18, 300–303 (2000). https://doi.org/10.1038/73745
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73745
This article is cited by
-
Artificial genetic polymers against human pathologies
Biology Direct (2022)
-
Synthetic molecular evolution of hybrid cell penetrating peptides
Nature Communications (2018)
-
MDM2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification
Nature Communications (2016)
-
PNAEμ can significantly reduce Burkitt's lymphoma tumor burden in a SCID mice model: cells dissemination similar to the human disease
Cancer Gene Therapy (2009)
-
Antiapoptotic fusion protein delivery systems
Macromolecular Research (2008)