Abstract
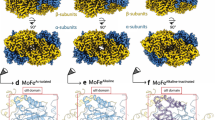
The crystal structure of the nitrogenase molybdenum–iron protein from Azotobacter vinelandii has been determined at 2.7 Å resolution. The α- and β-subunits in this α2 β2 tetramer have similar polypeptide folds. The FeMo-cofactor is completely encompassed by the α-subunit, whereas the P-cluster pair occurs at the interface between α- and β-subunits. Structural similarities are apparent between nitrogenase and other electron transfer systems, including hydrogenases and the photosynthetic reaction centre
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Burgess, B. K. in Advances in Nitrogen Fixation (eds Veeger, C. & Newton, W. E.) 103–114 (Martinus Nijhoff, Boston, 1984).
Orme-Johnson, W. H. A. Rev. Biophys. biophys. Chem. 14, 419–459 (1985).
Holm, R. H. & Simhon, E. D. in Molybdenum Enzymes (ed. Spiro, T. G.) Chapter 1 (Wiley-Interscience, New York, 1985).
Stiefel, E. I. et al. in Metal Clusters in Proteins (ed. Que, L.) 372–389 (American Chemical Society, Washington DC 1988).
Burgess, B. K. Chem. Rev. 90, 1377–1406 (1990).
Burris, R. H. J. biol. Chem. 266, 9339–9342 (1991).
Smith, B. E. & Eady, R. R. Eur. J. Biochem. 205, 1–15 (1992).
Simpson, F. B. & Burris, R. H. Science 224, 1095–1097 (1984).
Georgiadis, M. M. et al. Science 257, 1653–1659 (1992).
Brigle, K. E., Newton, W. E. & Dean, D. R. Gene 37, 37–44 (1985).
Kim, J. & Rees, D. C. Science 257, 1677–1682 (1992).
Lammers, P. J. & Haselkorn, R. Proc. natn. Acad. Sci. U.S.A. 80, 4723–4727 (1983).
Bowie, J. U., Lüthy, R. & Eisenberg, D. Science 253, 164–170 (1991).
Bishop, P. E. et al. in Nitrogen Fixation: Hundred Years After (eds Bothe, H., deBruijn, R. J. & Newton, W. E.) 71–79 (Gustav Fischer, Stuttgart, 1988).
Eady, R. R. Adv. inorg. Chem. 36, 77–102 (1991).
Brigle, K. E., Weiss, M. C., Newton, W. E. & Dean, D. R. J. Bact. 169, 1547–1553 (1987).
Robbins, A. H. & Stout, C. D. Proteins Struct. Funct Genet. 5, 289–312 (1989).
Wang, S.-Z., Chen, J.-S. & Johnson, J. L. Biochemistry 27, 2800–2810 (1988).
Yamane, T., Weininger, M. S., Mortenson, L. E. & Rossmann, M. G. J. biol. Chem. 257, 1221–1223 (1982).
Shah, V. K. & Brill, W. proc. natn. Acad. Sci. U.S.A. 74, 3249–3253 (1977).
Hawkes, T. R., McLean, P. A. & Smith, B. E. Biochem. J. 217, 317–321 (1984).
Adman, E. T., Watenpaugh, K. D. & Jensen, L. H. Proc. natn. Acad. Sci. U.S.A. 72, 4854–4858 (1975).
Govenzensky, D. & Zamir, A. J. Bact. 171, 5729–5735 (1989).
Wuttke, D. S., Bjerrum, M. J., Winkler, J. R. & Gray, H. B. Science 256, 1007–1009 (1992).
Case, D. A. & Karplus, M. J. molec. Biol. 132, 343–368 (1979).
Lowe, D. J. & Thorneley, R. N. F. Biochem. J. 215, 393–405 (1983).
Lowe, D. J. & Thorneley, R. N. F. Biochem. J. 224, 895–901 (1984).
Thorneley, R. N. F. in Nitrogen Fixation: Achievements and Objectives (eds Gresshoff, P. M., Roth, L. E., Stacey, G. & Newton, W. E.) 103–109 (Chapman and Hall, New York, 1990).
Feher, G., Allen, J. P., Okamura, M. Y. & Rees, D. C. Nature 339, 111–116 (1989).
Thomann, H., Bernardo, M., Newton, W. E. & Dean, D. R. Proc. natn. Acad. Sci. U.S.A. 88, 6620–6623 (1991).
Murrell, S. A., Lowery, R. G. & Ludden, P. W. Biochem. J. 251, 609–612 (1988).
Willing, A. & Howard, J. B. J. biol. Chem. 265, 6596–6599 (1990).
Thorneley, R. N. F., Ashby, G. A., Fisher, K. & Lowe, D. J. in Molybdenum Enzymes, Cofactors and Models (eds Stiefel, E., Coucouvanis, D. & Newton, W. E.) (American Chemical Society, Washington DC in the press).
Hadfield, K. L. & Bulen, W. A. Biochemistry 8, 5103–5108 (1969).
Adams, M. W. W. Biochim. biophys. Acta 1020, 115–145 (1990).
Deisenhofer, J., Epp, O., Miki, K., Huber, R. & Michel, H. Nature 318, 618–624 (1985).
Allen, J. P., Feher, G., Yeates, T. O., Komiya, H. & Rees, D. C. Proc. natn. Acad. Sci. U.S.A. 84, 5730–5734 (1987).
Terwilliger, T. C., Kim, S.-H. & Eisenberg, D. Acta crystallogr. A43, 1–5 (1987).
Rossmann, M. G. Molecular Replacement Method (Gordon and Breach, New York, 1972).
Bricogne, G. Acta crystallogr. A32, 832–847 (1976).
Jones, T. A. Meth. Enzym. 115, 151–171 (1985).
Tronrud, D. E., Ten Eyck, L. F. & Matthews, B. W. Acta crystallogr. A43, 489–501 (1987).
Brünger, A. T. J. Molec. Biol. 203, 803–816 (1988).
Carson, M. & Bugg, C. E. J. molec. Graphics 4, 121–122 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirn, J., Rees, D. Crystallographic structure and functional implications of the nitrogenase molybdenum–iron protein from Azotobacter vinelandii. Nature 360, 553–560 (1992). https://doi.org/10.1038/360553a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/360553a0
This article is cited by
-
Iron Nutrition Improves Productivity, Profitability, and Biofortification of Bread Wheat under Conventional and Conservation Tillage Systems
Journal of Soil Science and Plant Nutrition (2020)
-
The structure of vanadium nitrogenase reveals an unusual bridging ligand
Nature Chemical Biology (2017)
-
Highly efficient metal–organic-framework catalysts for electrochemical synthesis of ammonia from N2 (air) and water at low temperature and ambient pressure
Journal of Materials Science (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.