Abstract
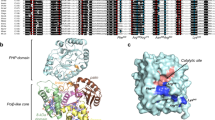
Escherichia coli contains three DNA polymerases that differ in their size, ability to interact with accessory proteins and biological function1. Monomeric DNA polymerase I (Pol I) has a relative molecular mass (Mr) of 103,000 (103K) and is involved primarily in the repair of damaged DNA and the processing of Okazaki fragments; polymerase II is of Mr 120K, and polymerase III has a Mr of 140K, is responsible for the replication of the DNA chromosome and is just one of several proteins that are required for replication. DNA polymerases from bacteriophage as well as those of eukaryotic viral and cellular origin also differ with respect to their size and the number of associated proteins that are required for them to function in replication1. However, the template-directed copying of DNA is identical in all cases. The crystal structure2 of the large proteolytic fragment of Pol I shows that it consists of two domains, the larger of which contains a deep crevice whose dimensions are such that it can bind duplex DNA. The T7 polymerase consists of two subunits, the 80K gene 5 protein and the host-encoded 12K thioredoxin of E. coli3,4. We show here that there is an amino acid sequence homology between at least eight polypeptide segments that form the large cleft in the Klenow fragment and polypeptides in T7 DNA polymerase gene 5 protein, suggesting that this domain evolved from a common precursor. The parts of the Pol I and T7 DNA polymerase molecules that bind the DNA substrate appear to share common structural features, and these features may be shared by all of these varied DNA polymerases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kornberg, A. DNA Replication (Freeman, San Francisco, 1980).
Ollis, D., Brick, P., Hamlin, R., Xuong, N. G. & Stetiz, T. A. Nature 313, 762–766 (1985).
Modrich, P. & Richardson, C. C. J biol. Chem. 250, 5515 (1975).
Mark, D. F. & Richardson, C. C. Proc. natn. Acad. Sci. U.S.A. 73, 780–784 (1976).
Dunn, J. J. & Sudier, F. W. J. molec. Biol. 166, 477–535 (1983).
Joyce, C. M., Kelley, W. S. & Grindley, N. D. F. J. biol. Chem. 257, 1958–1964 (1982).
McLachlan, A. D. J. molec. Biol. 61, 409–424 (1971).
Jones, T. H. J. appl. Crystallogr. 11, 265–272 (1978).
Kelly, W. S. & Grindley, N. D. F. Nucleic Acids Res. 3, 2971–2984 (1976).
Toh, H., Hayoshida, H. & Miyata, T. Nature 305, 827–829 (1983).
Ploegman, J. H., Drent, G., Kalk, K. H. & Hol, W. G. J. J. molec. Biol. 123, 557–594 (1978).
Dickerson, R. E. & Geis, I. Hemoglobin: Structure, Function, Evolution and Pathology, 77 (Benjamin/Cummings, Menlo Park, California, 1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ollis, D., Kline, C. & Steitz, T. Domain of E. coli DNA polymerase I showing sequence homology to T7 DNA polymerase. Nature 313, 818–819 (1985). https://doi.org/10.1038/313818a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/313818a0
This article is cited by
-
Pseudomonas aeruginosa phage PaP1 DNA polymerase is an A-family DNA polymerase demonstrating ssDNA and dsDNA 3′–5′ exonuclease activity
Virus Genes (2016)
-
A mechanism for initiating RNA-dependent RNA polymerization
Nature (2001)
-
Escherichia coli DNA polymerase II is homologous to α-like DNA polymerases
Molecular and General Genetics MGG (1991)
-
Genome organization of the linear plasmid, pSKL, isolated from Saccharomyces kluyveri
Molecular and General Genetics MGG (1991)
-
The kalilo linear senescence-inducing plasmid of Neurospora is an invertron and encodes DNA and RNA polymerases
Current Genetics (1991)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.