Abstract
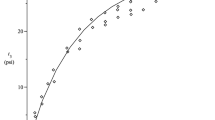
BIOLOGICAL fibres make a suitable subject for the development of the science of biomaterials. The amino-acid sequence of the collagen molecule is known1,2, and has been used to account for the self-assembly of collagen into native fibrils1 and polymorphic forms3, the pattern of bands due to positive staining in electron micrographs4 and the low-angle meridional X-ray diffraction pattern5. The collagen structure to amino acid resolution in axial projection is thus completely understood. Here we relate this structural picture to the mechanical properties (and since we are dealing with a biological fibre, these are also the biological properties) of the fibres. This can be done by investigating the vibrational and other motions of the molecular assembly in collagen. The force constants of hydrogen bonds in some polypeptides have been estimated on the basis of models6 but no experimental measure of their magnitude has been reported. We describe measurements of the inelastic light scattering (Brillouin) spectra of collagen from rat rail tendon from which we obtain elastic moduli of the tropocollagen molecule and an estimate of hydrogen bond force constants. We have also studied effects of hydration on the spectra to obtain information about the association of water with collagen in the native state. For comparison we have made preliminary measurements on molluscan ‘catch’ muscle in which the polypeptide conformation is different from collagen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hulmes, D. J. S., Miller, A., Parry, D. A. D., Piez, K. A. & Woodhead-Galloway, J. J. molec. Biol. 79, 137–148 (1972).
Fietzek, P. & Kuhn, K. Molec. cell. Biochem. 8, 141–157 (1975).
Doyle, B. B., Hukins, D. W. L., Hulmes, D. J. S., Miller, A. & Woodhead-Galloway, J. J. molec. Biol. 91, 79–99 (1975).
Doyle, B. B. et al. Proc. R. Soc. B 187, 34–46 (1974).
Hulmes, D. J. S., Miller, A., White, S. & Doyle, B. B. J. molec. Biol. 110, 643–666 (1977).
Fanconi, B. & Peticolas, W. L. Biopolymer 10, 2223–2229 (1971).
Fabelinskii, I. L. in Molecular Scattering of Light (Plenum, New York, 1968).
Chothia, C. H. Nature 254, 304–308 (1975).
Bear, R. S. J. biophys. biochem. Cytol. 2, 363 (1956).
Rougvie, A. A. & Bear, R. S. J. Am. Leather Chem. As. 48, 735–751 (1953).
Holliday, L. & White, J. W. Pure appl. Chem. 26, 545–582 (1971).
White, J. W. Brookhaven Symp. Biol. 27, VI–3 to VI–24 (1975).
Cowan, P. M., North, A. C. T. & Randall, J. T. Symp. Soc. exp. Biol. 9, 115–126 (1955).
McCutchen, C. W. theoretical Biol. 51, 51–58 (1975).
Tristram, G. R. & Smith, R. H. in The Proteins 1 (ed. Neurath, H.), 45–57 (Academic, New York, 1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HARLEY, R., JAMES, D., MILLER, A. et al. Phonons and the elastic moduli of collagen and muscle. Nature 267, 285–287 (1977). https://doi.org/10.1038/267285a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/267285a0
This article is cited by
-
Assessment of myocardial viscoelasticity with Brillouin spectroscopy in myocardial infarction and aortic stenosis models
Scientific Reports (2021)
-
Mapping mechanical properties of biological materials via an add-on Brillouin module to confocal microscopes
Nature Protocols (2021)
-
Mechanical Properties of cellulose fibers measured by Brillouin spectroscopy
Cellulose (2020)
-
An efficient two-scale 3D FE model of the bone fibril array: comparison of anisotropic elastic properties with analytical methods and micro-sample testing
Biomechanics and Modeling in Mechanobiology (2020)
-
Multiscale modeling of human bone
Multiscale and Multidisciplinary Modeling, Experiments and Design (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.