Abstract
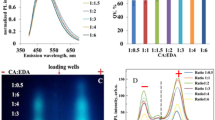
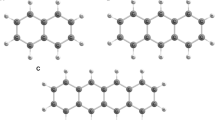
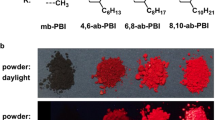
IN contrast with the characteristic fluorescence spectrum (Curve 1) which 3: 4,benzpyrene always emits when it is dissolved in solvents, we have observed three different types of fluorescent spectra (Curves 2, 3, 4) when the hydrocarbon was excited in the solid state. The needles of a commercial benzpyrene (La Roche) emit a green fluorescent light and we call it therefore the ‘green’ form. Curve 2 exhibits a broad rather symmetrical band with its flat maximum between 500 and 510 m.μ. On pouring an acetone solution of the green form of benzpyrene into cold water there results the well-known colloidal suspension of benzpyrene which emits a yellowish fluorescence. The spectrogram of this ‘yellow’ form has its maximum between 530 m.μ and 540 m.μ, and a typical inflection at the short wave-length side of the band (Curve 3). On heating the green form in an evacuated tube, a white sublimate is formed on the tube walls which fluoresces brightly blue. The spectrogram of this ‘blue’ form shows a maximum between 445 m.μ and 450 mμ, and an inflection on the short-wave-length side which appears as a contrast band at about 425 m.μ (Curve 4). The sublimed crystals are small plates with typically curved edges. We believe that they are identical with the modification of benzpyrene which Iball2 obtained from a solution in amyl acetate. According to his X-ray analysis, they are orthorhombic, while the needles (our green form) belong to the monoclinic system. Iball did not examine the fluorescence of either form.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Goldberg, E., Z. Reprod. Techn., No. 4 (1910).
Iball, J., Z. Kryst, A, 94, 7 (1936).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WEIGERT, F., MOTTRAM, J. Some Physico-Chemical Properties of 3:4,Benzpyrene. Nature 145, 895–896 (1940). https://doi.org/10.1038/145895a0
Issue Date:
DOI: https://doi.org/10.1038/145895a0
This article is cited by
-
Die polymorphic von benzo[a]pyren
Naturwissenschaften (1978)
-
Absorption Spectra of 3-4 Benzpyrene
Nature (1942)
-
Transformation of Benzpyrene in the Living Skin of Mice into a Compound Soluble in Dilute Alkali
Nature (1942)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.