Abstract
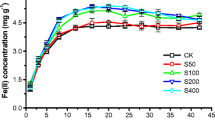
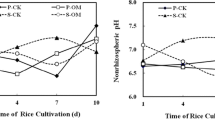
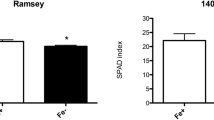
Loading of extraplasmatic Fe, as a potential storage pool for Fe nutrition, was studied in roots of maize grown under hypoxic conditions in soil culture. Extraplasmatic Fe loading was investigated depending on (i) duration of flooding (0, 1, 2 or 4 days) and (ii) microbial activity as affected by graduated addition of carbon sources (0, 2 or 10 g each starch and cellulose kg−1 soil). Maize plants were grown in a soil culture system with root systems enclosed in membrane bags to avoid Fe contamination of the root surface by soil particles. Due to the high redox buffer capacity of the Haplic Luvisol employed for the experiments, flooding treatments induced only moderately reducing conditions (∼ 300 mV) and a slight increase of extraplasmatic Fe loading (41\to165 mg kg−1 d.m.). Strongly reducing conditions (−100 mV) associated with a high Fe2+ concentration in the soil solution and a significant increase of extraplasmatic Fe (1190 mg kg−1 d.m.) were obtained only after application of high amounts of organic carbon (10 g starch and 10 g cellulose kg−1 soil), which accompanied by unrealistic reducing conditions due to intense stimulation of microbial growth. The expression of effects only under extremely high application level of organic carbon (∼ 33 t C ha−1) suggest that similar to aerobic conditions, extraplasmatic Fe-loading under transient hypoxia is probably of limited ecological significance for the iron nutrition of higher plants, at least in soils with a high redox buffer capacity as employed in the present study.
Abbreviations: DHA – dehydrogenase activity; d.m. – dry matter; DOC – dissolved organic carbon; Eh – redox potential; PIXE – proton-induced X-ray emission; STIM – scanning transmission ion microscopy.
Similar content being viewed by others
References
Armstrong W 1979 Aeration in higher plants. Adv. Bot. Res. 7, 226–332.
Bartlett R J and James B R 1993 Redox chemistry of soils. Adv. Agron. 50, 151–208.
Begg C B M, Kirk G J D, Mackenzie A H and Neue H-U 1994 Root-induced iron oxidation and pH changes in the lowland rice rhizoshere. New Phytol. 128, 469–477.
Bienfait H F, Van den Briel W and Mesland-Mul N T 1985 Free space iron pools in roots: Generation and mobilization. Plant Physiol. 78, 596–600.
Borggaard O K 1992 Dissolution of poorly crystalline iron oxides in soils by EDTA and oxalate. Z. Pflanzenernaehr. Bodenkd. 155, 431–436.
Brzezinska M, Stepniewska Z and Stepniewski W1995 Soil oxygen status and dehydrogenase activity. Soil Biol. Biochem. 30, 1783–1790.
Drew M C, Chamel A, Garrec J P and Fourcy A 1980 Cortical air space (aerenchyma) in roots of corn subjected to oxygen stress. Plant Physiol. 65, 506–511.
FAO-WRB 1998 World reference base for soil resources. Food and Agriculture Organization of the United Nations. Rome, Italy.
Farrell R E, Swerhone G D W and Van Kessel C 1991 Construction and evaluation of a reference electrode assembly for use in monitoring in situ soil redox potentials. Commun. Soil. Sci. Plant Anal. 22, 1059–1068.
Fiedler S 1997 In-situ-Langzeitmessungen des Redoxpotentials in hydromorphen Böden einer Endmoränenlandschaft im württembergischen Alpenvorland. Hohenheimer Bodenkd. Hefte 42, 135 p.
Flessa H and Fischer W R 1992 Plant-induced changes in the redox potentials of rice rhizospheres. Plant Soil 143, 55–60.
Gibbs J, de Bruxelle G, Armstrong W and Greenway H 1995 Evidence for anoxic zones in 2–3 mm tips of aerenchymatous maize roots under low O 2 supply. Aust. J. Plant Physiol. 22, 723–730.
Jenkinson D S, Davidson S A and Powlson D S 1979 Adenosine triphosphate and microbial biomass in soil. Soil Biol. Biochem. 11, 521–527.
Johnson J, Cobb B G and Drew M C 1989 Hypoxic induction of anoxia tolerance in root tips of Zea mays. Plant Physiol. 91, 837–841.
Kalbitz K, Solinger S, Park J-H, Michalzik B and Matzner E 2000 Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 165, 277–304.
Kludze H K and DeLaune R D 1996 Soil redox intensity effects on oxygen exchange and growth of Cattail and sawgrass. Soil Sci. Soc. Am. J. 60, 616–620.
Lehmann R G, Cheng H H and Harsh J B 1987 Oxidation of phenolic acids by soil iron and manganese oxides. Soil Sci. Soc. Am. J. 51, 352–356.
Longnecker N and Welch R M 1990 Accumulation of apoplastic iron in plant roots. Plant Physiol. 92, 17–22.
Marschner H 1995 Mineral nutrition of higher plants. Academic Press, London.
Masalha J, Kosegarten H, Elmaci O and Mengel K 1999 The central role of microbial activity for iron acquisition in maize and sunflower. Biol. Fertil. Soils. 30, 433–439.
Mengel K 1994 Iron availabilty in plant tissues – Iron clorosis on calcareous soils. Plant Soil 165, 275–283.
Mulholland P J, Dahm C N, David B M, DiToro D M, Fisher T R, Kögel-Knabner I, Meybeck M H, Meyer J L and Sedell J R 1990 What are the temporal and spatial variations of organic acids at the ecosystem level? In Organic Acids in Aquatic Ecosystems. Eds. E M Perdue and E T Gjessing. pp. 315–329 Life Sciences Research Report 48. John Wiley and Sons, Chichester.
Neumann G and Römheld V 2000 The release of root exudates as affected by the plant's physiological status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface. Eds. R Pinton, Z Varanini and P Nannipieri. pp. 41–93. Marcel Dekker, New York.
Otsuki A and Hanya T 1972 Production of dissolved organic matter from dead green alga cells. II. Anaerobic microbial decomposition. Limnol. Oceanogr. 17, 258–264.
Pezeshki S R, Matthews S W and DeLaune R D 1991 Root cortex structure and metabolic responses of Spartina patens to soil redox conditions. Environ. Exp. Bot. 31, 91–97.
Ponnamperuma F N 1984 The effects of flooding on soils. In Flooding and Plant Growth. Ed. T T Kozlowski. pp. 9–45. Academic Press, London.
Saglio P H, Drew M C and Pradet A 1988 Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pre-treatment (Hypoxia) in root tips of Zea mays. Plant Physiol. 86, 61–66.
Santiago l S, Goldstein G, Meinzer F C, Fownes J H, Mueller-Dombois D 2000 Transpiration and forest structure in relation to water logging in a Hawaiian mountain cloud forest. Tree Physiol. 20, 673–681.
SAS 1989 Statistical Analysis System, Release 6.09, SAS Institute Inc., Cary NC, USA.
Satpathy S N, Rath A K, Ramakrishnan B, Rao V R, Adhya T K and Sethunathan N 1997 Diurnal variation in methane flux at different growth stages of tropical. Plant Soil 195, 267–271.
Sattelmacher B, Mühling K H and Pennewiss K 1998 The apoplast – Its significance for the nutrition of higher plants. Z. Pflanzenernaehr. Bodenkd. 161, 485–198.
Schilt A A and Hoyle W C 1967 Improved sensitivity and selectivity in the spectrophotometric determination of iron by use of a new ferrozin-type reagent. Anal. Chem. 39, 114–117.
Schinner F, Öhlinger R, Kandeler E and Margesin R 1993 Boden-biologische Arbeitsmethoden. pp. 60–62 and 122–124. Springer, Berlin-Heidelberg.
Schlichting E, Blume H P and Stahr K 1995 Bodenkundliches Praktikum. Eine Einführung in pedologische Arbeiten für Ökologen, insb. Land-und Forstwirte, und für Geowissenschaftler. Blackwell, Berlin Wien. 295 pp.
Schmidt W 1999 Review Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 141, 1–26.
Schneider T, Povh B, Strasser O, Gierth M, Przybylowicz W, Mesjasz-Przybylowicz J, Churms C and Schüßler A 1999 Micro-Pixe evaluation of Fe distribution in barley roots. Intern. J. PIXE 9, 353–364.
Schneider T, Strasser O, Gierth M, Scheloske S and Povh B 2001 Micro-pixe investigations of apoplastic Fe in freezed dried root cross sections of soil grown barley. Nucl. Inst. Meth. Phys. Res. – B Beam Interactions with Material and Atoms 189, 487–793.
Snowden R E D and Wheeler B D 1995 Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytol. 131, 503–520.
Strasser O. 2000 Bedeutung des Wurzelapoplasten als Eisenspeicher für Pflanzen. Grauer Beuren, Stuttgart. 152 pp.
Strasser O, Köhl K and Römheld V 1999 Overestimation of apoplastic Fe in roots of soil grown plants. Plant Soil 210, 179–187.
Thalmann A 1968 Zur Methodik der Bestimmung der Dehydrogeneseaktivität im Boden mittels Triphenyltetrazoliumchlorid. Landwirt. Forsch. 21, 249–258.
Trolldenier G 1988 Visualisation of oxidising power of rice roots and of possible participation of bacteria in iron deposition. Z. Pflanzenernaehr. Bodenkd. 151, 117–121.
Von der Kammer F, Thöming J and Förster U 2000 Redox buffer capacity concept as a tool for the assessment of long-term effects in natural attenuation/intrinsic remediation. In Redox – Fundamentals, Processes and Application. Eds. J Schüring, H D Schulz, W R Fischer, J Böttcher and W H M Duijnisveld. pp. 189–202. Springer, Berlin, Heidelberg, New York.
Von Wirén N, Römheld V, Shioiri S and Marschner H 1995 Iron Competition between microorganism and root of barley and sorghum for iron accumulation in the root apoplasm. New Phytol. 130, 511–521.
Yan B, Dai Q, Liu X, Huang S and Wang Z 1996 Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179, 261–268.
Zhang F S, Römheld V and Marschner H 1991 Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol. 97, 1302–1350.
Zhang X, Cuiling Y I and Zhang F 1999 Iron accumulation in root apoplasm of dicotyledonous and graminaceous species grown on calcareous soil. New Phytol. 141, 27–31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fiedler, S., Strasser, O., Neumann, G. et al. The influence of redox conditions in soils on extraplasmatic Fe-loading of plant roots. Plant Soil 264, 159–169 (2004). https://doi.org/10.1023/B:PLSO.0000047755.53388.31
Issue Date:
DOI: https://doi.org/10.1023/B:PLSO.0000047755.53388.31