Abstract
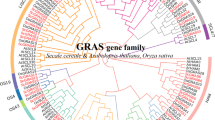
Members of the GRAS gene family encode transcriptional regulators that have diverse functions in plant growth and development such as gibberellin signal transduction, root radial patterning, axillary meristem formation, phytochrome A signal transduction, and gametogenesis. Bioinformatic analysis identified 57 and 32 GRAS genes in rice and Arabidopsis, respectively. Here, we provide a complete overview of this gene family, describing the gene structure, gene expression, chromosome localization, protein motif organization, phylogenetic analysis, and comparative analysis between rice and Arabidopsis. Phylogenetic analysis divides the GRAS gene family into eight subfamilies, which have distinct conserved domains and functions. Both genome/segmental duplication and tandem duplication contributed to the expansion of the GRAS gene family in the rice and Arabidopsis genomes. The existence of GRAS-like genes in bryophytes suggests that GRAS is an ancient family of transcription factors, which arose before the appearance of land plants over 400 million years ago.
Similar content being viewed by others
References
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Arabidopsis Genome Initiative. 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815.
Bailey, T.L. and Elkan, C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2: 28–36.
Baumberger, N., et al. 2003. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade([w]). Plant Physiol. 131: 1313–1326.
Blanc, G., Barakat, A., Guyot, R., Cooke, R. and Delseny, M. 2000. Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12: 1093–1101.
Blanc, G., Hokamp, K. and Wolfe, K.H. 2003. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res. 13: 137–144.
Bolle, C., Koncz, C. and Chua, N.-H. 2000. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 14: 1269–1278.
Bowers, J.E., Chapman, B.A., Rong, J. and Paterson, A.H. 2003. Unraveling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422: 433–438.
Chen, M., et al. 2002. An integrated physical and genetic map of the rice genome. Plant Cell 14: 537–545.
Chomczynski, P. and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal. Biochem. 162: 156–159.
Day, R.B., Shibuya, N. and Minami, E. 2003. Identification and characterization of two new members of the GRAS gene family in rice responsive to N-acetylchitooligosaccharide elicitor. Biochim. Biophys. Acta 1625: 261–268.
Di Laurenzio, L., Wysocka-Diller, J., Malamy, J.E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M.G., Feldmann, K.A. and Benfey, P.N. 1996. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86: 423–433.
Dill, A., Jung, H.S. and Sun, T.P. 2001. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA 98: 14162–14167.
Dingwall, C. and Laskey, R.A. 1991. Nuclear targeting sequences: a consensus? Trends Biochem. Sci. 16: 478–481.
Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5: 164–166.
Feng, Q., et al., 2002. Sequence and analysis of rice chromosome 4. Nature 420: 316–320.
Fu, X., Richards, D.E., Ait-Ali, T., Hynes, L.W., Ougham, H., Peng, J. and Harberd, N.P. 2002. Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14: 3191–3200.
Gagne, J.M., Downes, B.P., Shiu, S.H., Durski, A.M. and Vierstra, R.D. 2002. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. USA 99: 11519–11524.
Goff, S.A., et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100.
Greb, T., Clarenz, O., Schafer, E., Muller, D., Herrero, R., Schmitz, G. and Theres, K. 2003. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 17: 1175–1187.
Harushima, Y. et al. 1998. A high-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics 148: 479–494.
Heery, D.M., Kalkhoven, E., Hoare, S. and Parker, M.G. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387: 733–736.
Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M.T. and Benfey, P.N. 2000. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101: 555–567.
Huala, E., et al. 2001. The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based informa-tion retrieval, analysis, and visualization system for a model plant. Nucl. Acids Res. 29: 102–105.
Ikeda, A., Sonoda, Y., Vernieri, P., Perata, P., Hirochika, H. and Yamaguchi, J. 2002. The slender rice mutant, with constitutively activated gibberellin signal transduction, has enhanced capacity for abscisic acid level. Plant Cell Physiol. 43: 974–979.
Ikeda, A., Ueguchi-Tanaka, M., Sonoda, Y., Kitano, H., Koshioka, M., Futsuhara, Y., Matsuoka, M. and Yamaguchi, J. 2001. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13: 999–1010.
Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M. and Matsuoka, M. 2002. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14: 57–70.
Kikuchi, S., et al. 2003. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301: 376–379.
Ku, H.M., Vision, T., Liu, J. and Tanksley, S.D. 2000. Comparing sequenced segments of the tomato and Arabidopsis genomes: large-scale duplication followed by selective gene loss creates a network of synteny. Proc. Natl. Acad. Sci. USA 97: 9121–9126.
Kumar, S., Tamura, K., Jakobsen, I.B. and Nei, M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17: 1244–1245.
Lee, S., Cheng, H., King, K.E., Wang, W., He, Y., Hussain, A., Lo, J., Harberd, N.P. and Peng, J. 2002. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following inhibition. Genes Dev. 16: 646–658.
Li, X., et al. 2003. Control of tillering in rice. Nature 422: 618–621.
Liu, L., White, M.J. and MacRae, T.H. 1999. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur. J. Biochem. 262: 247–257.
Lim, J., Helariutta, Y., Specht, C.D., Jung, J., Sims, L., Bruce, W.B., Diehn, S. and Benfey, P.N. 2000. Molecular analysis of the SCARECROW gene in maize reveals a common basis for radial patterning in diverse meristems. Plant Cell 12: 1307–1318.
Lynch, M. and Conery, J.S. 2000. The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155.
Morohashi, K., Minami, M., Takase, H., Hotta, Y. and Hiratsuka, K. 2003. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J. Biol. Chem. 278: 20865–20873.
Muse, S.V. 2000. Examining rates and patterns of nucleotide substitution in plants. Plant Mol. Biol. 42: 25–43.
Nei, M. and Gojobori, T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3: 418–426.
Nishiyama, T., et al. 2003. Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc. Natl. Acad. Sci. USA 100: 8007–8012.
Paterson, A., Bowers, J., Peterson, D., Estill, J. and Chapman, B. 2003. Structure and evolution of cereal genomes. Curr. Opin. Genet. Dev. 13: 644–650.
Peng, J., Carol, P., Richards, D.E., King, K.E., Cowling, R.J., Murphy, G.P. and Harberd, N.P. 1997. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 11: 3194–3205.
Peng, J., et al. 1999. 'Green revolution' genes encode mutant gibberellin response modulators. Nature 400: 256–261.
Pysh, L.D., Wysocka-Diller, J.W., Camilleri, C., Bouchez, D. and Benfey, P.N. 1999. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 18: 111–119.
Rice Chromosome 10 Sequencing Consortium. 2003. In-depth view of structure, activity, and evolution of rice chromosome 10. Science 300: 1566–1569.
Robbins, J., Dilworth, S.M., Laskey, R.A. and Dingwall, C. 1991. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64: 615–623.
Salamov, A.A. and Solovyev, V.V. 2000. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 10: 516–522.
Sasaki, T., et al. 2002. The genome sequence and structure of rice chromosome 1. Nature 420: 312–316.
Sassa, N., Matsushita, Y., Nakamura, T. and Nyunoya, H. 2001. The molecular characterization and in situ expression pattern of pea SCARECROW gene. Plant Cell Physiol. 42: 385–394.
Schumacher, K., Schmitt, T., Rossberg, M., Schmitz, G. and Theres, K. 1999. The Lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl. Acad. Sci. USA 96: 290–295.
Silverstone, A.L., Ciampaglio, C.N. and Sun, T. 1998. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10: 155–169.
Simillion, C., Vandepoele, K., Van Montagu, M., Zabeau, M. and Van de Peer, Y. 2002. The hidden duplication past of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 99: 13627–13632.
Stuurman, J., Jaggi, F. and Kuhlemeier, C. 2002. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes Dev. 16: 2213–2218.
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. and Higgins, D.G. 1997. The CLUSTAL_X windows interface: .exible strategies for multiple sequence alignment aided by quality analysis tools.
Torchia, J., Rose, D.W., Inostroza, J., Kamei, Y., Westin, S., Glass, C.K. and Rosenfeld, M.G. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function.Nature 387: 677-684.
Triezenberg, S.J. 1995. Structure and function of transcriptional activation domains. Curr. Opin. Genet. Dev. 5: 190
Vandepoele, K., Simillion, C. and Van de Peer, Y. 2003. Evidence that rice and other cereals are ancient aneuploids. Plant Cell 15: 2192–2202.
Varagona, M.J., Schmidt, R.J. and Raikhel, N.V. 1992. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell 4: 1213–1227.
Vision, T.J., Brown, D.G. and Tanksley, S.D. 2000. The origins of genomic duplications in Arabidopsis. Science 290: 2114-2117. 531 Wen, C.K. and Chang, C. 2002. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87100.
Wikstrom, N., Savolainen, V. and Chase, M.W. 2001. Evolution of the angiosperms: calibrating the family tree. Proc. R. Soc. Lond. B. Biol. Sci. 268: 2211–2220.
Wolfe, K.H. 2001. Yesterday's polyploids and the mystery of diploidization. Nat. Rev. Genet. 2: 333–341.
Wolfe, K.H., Gouy, M., Yang, Y.W., Sharp, P.M. and Li, W. 1989. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc. Natl. Acad. Sci. USA 86: 6201–6205.
Wu, J., Kurata, N., Tanoue, H., Shimokawa, T., Umehara, Y., Yano, M. and Sasaki, T. 1998. Physical mapping of duplicated genomic regions of two chromosome ends in rice. Genetics 150: 1595–1603.
Yang, Z. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13: 555–556.
Yang, Z. and Nielsen, R. 2000. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 17: 32–43.
Yu, J., et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92.
Author information
Authors and Affiliations
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tian, C., Wan, P., Sun, S. et al. Genome-Wide Analysis of the GRAS Gene Family in Rice and Arabidopsis . Plant Mol Biol 54, 519–532 (2004). https://doi.org/10.1023/B:PLAN.0000038256.89809.57
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000038256.89809.57