Abstract
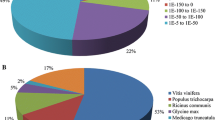
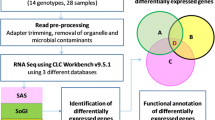
Sugarcane accumulates high concentrations of sucrose in the mature stem and a number of physiological processes on-going in maturing stem tissue both directly and indirectly allow this process. To identify transcripts that are associated with stem maturation, we compared patterns of gene expression in maturing and immature stem tissue by expression profiling and bioinformatic analysis of sets of stem ESTs. This study complements a previous study of gene expression associated directly with sugar metabolism in sugarcane. A survey of sequences derived from stem tissue identified an abundance of several classes of sequence that are associated with fibre biosynthesis in the maturing stem. A combination of EST analyses and microarray hybridization revealed that genes encoding homologues of the dirigent protein, a protein that assists in the stereospecificity of lignin assembly, were the most abundant and most strongly differentially expressed transcripts in maturing stem tissue. There was also evidence of coordinated expression of other categories of fibre biosynthesis and putative defence- and stress-related transcripts in the maturing stem. This study has demonstrated the utility of genomic approaches using large-scale EST acquisition and microarray hybridization techniques to highlight the very significant transcriptional investment the maturing stem of sugarcane has placed in fibre biosynthesis and stress tolerance, in addition to its already well-documented role in sugar accumulation.
Similar content being viewed by others
References
Allona, I., Quinn, M., Shoop, E., Swope, K., Stcyr, S., Carlis, J., Riedl, J., Retzel, E., Campbell, M.M., Sederoff, R. and Whetten, R.W. 1998. Analysis of xylem formation in pine by cDNA sequencing. Proc. Natl. Acad. Sci. USA 95: 9693–9698.
Altschul, S.F., Gish, W., Miller, W., Meyers, E.W. and Lipman, D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410.
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Artschwager, E. 1925. Anatomy of the vegetative organs of sugar cane. J. Agric. Res. 30: 197–241.
Bernards, M.A. 2002. Demystifying suberin. Can. J. Bot. 80: 227–240.
Botha, F.C. and Black, K.G. 2000. Sucrose phosphate synthase and sucrose synthase activity during maturation of internodal tissue in sugarcane. Aust. J. Plant Physiol. 27: 81–85.
Bull, T.A. and Glasziou, K.T. 1963. The evolutionary significance of sugar accumulation in Saccharum. Aust. J. Biol. Sci. 16: 737–742.
Burlat, V., Kwon, M., Davin, L.B. and Lewis, N.G. 2001. Dirigent proteins and dirigent sites in lignifying tissues. Phytochemistry 57: 883–897.
Carson, D.L., Huckett, B.I. and Botha, F.C. 2002. Sugarcane ESTs differentially expressed in immature and maturing internodal tissue. Plant Sci. 162: 289–300.
Casu, R.E., Grof, C.P.L., Rae, A.L., McIntyre, C.L., Dimmock, C.M. and Manners, J.M. 2003. Identification of a novel sugar transporter homologue strongly expressed in maturing stem vascular tissues of sugarcane by expressed sequence tag and microarray analysis. Plant Mol. Biol. 52: 371–386.
Covitz, P.A., Smith, L.S. and Long, S.R. 1998. Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiol. 117: 1325–1332.
Davin, L.B., Wang, H.B., Crowell, A.L., Bedgar, D.L., Martin, D.M., Sarkanen, S. and Lewis, N.G. 1997. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 275: 362–366.
Fernandes, J., Brendel, V., Gai, X., Lal, S., Chandler, V.L., Elumalai, R.P., Galbraith, D.W., Pierson, E.A. and Walbot, V. 2002. Comparison of RNA expression profiles based on maize expressed sequence tag frequency analysis and microarray hybridization. Plant Physiol. 128: 896–910.
Gang, D.R., Costa, M.A., Fujita, M., Dinkova-Kostova, A.T., Wang, H.B., Burlat, V., Martin, W., Sarkanen, S., Davin, L.B. and Lewis, N.G. 1999. Regiochemical control of monolignol radical coupling: a new paradigm for lignin and lignan biosynthesis. Chem. Biol. 6: 143–151.
Held, B.M., Wang, H., John, I., Wurtele, E.S. and Colbert, J.T. 1993. An mRNA putatively coding for an O-methyltransferase accumulates preferentially in maize roots and is located predominantly in the region of the endodermis. Plant Physiol. 102: 1001–1008.
Ihaka, R. and Gentleman, R. 1996. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 5: 299–314.
Jacobsen, K.R., Fisher, D.G. and Moore, P.H. 1992. Developmental changes in the anatomy of the sugarcane stem in relation to phloem unloading and sucrose storage. Bot. Acta 105: 70–80.
Keating, B.A., Robertson, M.J., Muchow, R.C. and Huth, N.I. 1999. Modelling sugarcane production systems. I. Development and performance of the sugarcane module. Field Crops Res. 61: 253–271.
Kim, M.K., Jeon, J.H., Davin, L.B. and Lewis, N.G. 2002a. Monolignol radical-radical coupling networks in western red 516 cedar and Arabidopsis and their evolutionary implications. Phytochemistry 61: 311–322.
Kim, M.K., Jeon, J.H., Fujita, M., Davin, L.B. and Lewis, N.G. 2002b. The western red cedar (Thuja plicata) 8-8' DIRIGENT family displays diverse expression patterns and conserved monolignol coupling specificity. Plant Mol. Biol. 49: 199–214.
Klok, E.J., Wilson, I.W., Wilson, D., Chapman, S.C., Ewing, R.M., Somerville, S.C., Peacock, W.J., Dolferus, R. and D ennis, E.S. 2002. Expression profile analysis of the lowoxygen response in Arabidopsis root cultures. Plant Cell 14: 2481–2494.
Kolattukudy, P.E. 1984. Biochemistry and function of cutin and suberin. Can. J. Bot. 62: 2918–2933.
Lange, B.M., Lapierre, C. and Sandermann, H. 1995. Elicitorinduced spruce stress lignin (structural similarity to early developmental lignins). Plant Physiol. 108: 1277–1287.
Muchow, R.C., Robertson, M.J. and Wood, A.W. 1996. Growth of sugarcane under high input conditions in tropical Australia. II. Sucrose accumulation and commercial yield. Field Crops Res. 48: 27–36.
Reichheld, J.P., Sonobe, S., Clement, B., Chaubet, N. and Gigot, C. 1995. Cell cycle-regulated histone gene expression in synchronized plant cells. Plant J. 7: 245–252.
Shi, H., Quintero, F.J., Pardo, J.M. and Zhu, J.K. 2002. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14: 465–477.
Sterky, F., Regan, S., Karlsson, J., Hertzberg, M., Rohde, A., Holmberg, A., Amini, B., Bhalerao, R., Larsson, M., Villarroel, R., van Montagu, M., Sandberg, G., Olsson, O., Teeri, T.T., Boerjan, W., Gustafsson, P., Uhlen, M., Sundberg, B. and Lundeberg, J. 1998. Gene discovery in the woodforming tissues of poplar: analysis of 5692 expressed sequence tags. Proc. Natl. Acad. Sci. USA 95: 13330–13335.
Szekeres, M., Haizel, T., Adam, E. and Nagy, F. 1995. Molecular characterization and expression of a tobacco histone H1 cDNA. Plant Mol. Biol. 27: 597–605.
Vettore, A.L., da Silva, F.R., Kemper, E.L. and Arruda, P. 2001. The libraries that made SUCEST. Gen. Mol. Biol. 24: 1–7.
Wang, Y., Nowak, G., Culley, D., Hadwiger, L.A. and Fristensky, B. 1999. Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus). Mol. Plant-Microbe Interact. 12: 410–418.
Welbaum, G.E. and Meinzer, F.C. 1990. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol. 93: 1147–1153.
Whittaker, A. and Botha, F.C. 1997. Carbon partitioning during sucrose accumulation in sugarcane internodal tissue. Plant Physiol. 115: 1651–1659.
Wilson, D.L., Buckley, M.J., Helliwell, C.A. and Wilson, I.W. 2003. New normalization methods for cDNA microarray data. Bioinformatics 19: 1325–1332.
Yang, Y.H., Dudoit, S., Luu, P. and Speed, T.P. 2001. Normalization for cDNA microarray data. http://www. stat.berkeley.edu/users/terry/zarray/Html/normspie.html.
Zeier, J. and Schreiber, L. 1998. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta 206: 349–361.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casu, R.E., Dimmock, C.M., Chapman, S.C. et al. Identification of Differentially Expressed Transcripts from Maturing Stem of Sugarcane by in silico Analysis of Stem Expressed Sequence Tags and Gene Expression Profiling. Plant Mol Biol 54, 503–517 (2004). https://doi.org/10.1023/B:PLAN.0000038255.96128.41
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000038255.96128.41