Abstract
Purpose. To study the effect of solution conditions on the structural conformation of recombinant human interferon-α2a (IFNα2a) to investigate its tendency to form partially unfolded intermediates.
Methods. The structural properties of IFNα2a were studied at various pH values (2.0-7.4) and temperatures (5°C-80°C) using Trp fluorescence emission, fluorescence quenching, near- and far-UV circular dichroism (CD) spectroscopy, and DSC.
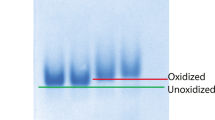
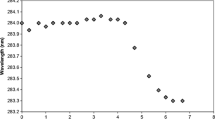
Results. Fluorescence intensity measurements as a function of temperature indicated the onset of the thermal unfolding of IFNα2a, denoted by Td, around 60°C above pH 4.0. Td was not observed at pH 3.5 and below. Acrylamide and iodide quenching studies indicated partial unfolding of protein with decrease in pH and with increase in temperature up to 50°C. Near-UV CD studies indicated a significant loss in the tertiary structure of protein on increase in temperature from 15°C to 50°C at all solution pHs. DSC scans supported results obtained from fluorescence and CD studies at pH 4.0 and below. DSC, however, was insensitive to changes that occurred at moderate temperatures at pH 5.0 and 7.4.
Conclusions. IFNα2a has a tendency to acquire multiple partially unfolded states with structural conformations sensitive to solution pH and temperature. These states were formed at moderate temperatures, and it is speculated that these partially unfolded states could play an important role in the aggregation of proteins during the long-term storage of aqueous protein formulations.
Similar content being viewed by others
References
M. C. Manning, K. Patel, and R. T. Borchardt. Stability of protein pharmaceuticals. Pharm. Res. 6:903-918 (1989).
T. Arakawa, S. Prestrelski, W. C. Kenney, and J. F. Carpenter. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 10:1-28 (1993).
W. Wang. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 185:129-188 (1999).
O. B. Ptitsyn. Molten globule and protein folding. Adv. Prot. Chem. 47:83-229 (1995).
A. L. Fink. Compact intermediate states in protein folding. Annu. Rev. Biophys. Biomol. Struct. 24:495-522 (1995).
K. Kuwajima. The molten globule as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins 6:87-103 (1989).
C. M. Dobson. Unfolded proteins, compact states and molten globules. Curr. Opin. Struct. Biol. 2:6-12 (1992).
V. N. Uversky, N. V. Narizhneva, S. O. Kirschstein, S. Winter, and G. Lober. Conformational transitions provoked by organic solvents in β-lactoglobulin: can a molten globule like intermediate be induced by the decrease in dielectric constant. Folding Design 2:163-172 (1997).
K. S. Vassilenko and V. N. Uversky. Native-like secondary structure of molten globules. Biochim. Biophys. Acta 1594:168-177 (2002).
J. E. Matsuura, A. E. Morris, R. R. Ketchem, E. H. Braswell, R. Klinke, W. R. Gombotz, and R. L. Remmele, Jr. Biophysical characterization of a soluble CD40 ligand (CD154) coiled-coil trimer: Evidence of a reversible acid-denatured molten globule. Arch. Biochem. Biophys. 392:208-218 (2001).
T. Koshiba, M. Yao, Y. Kobashigawa, M. Demura, A. Nakagawa, I. Tanaka, K. Kuwajima, and K. Nitta. Structure and thermodynamics of the extraordinarily stable molten globule state of canine milk lysozyme. Biochemistry 39:3248-3257 (2000).
S. Wicar, M. G. Mulkerrin, G. Bathory, L. H. Khundkar, and B. L. Karger. Conformational changes in the reversed phase liquid chromatography of recombinant human growth hormone as a function of organic solvent: The molten globule state. Anal. Chem. 66:3908-3915 (1994).
O. B. Ptitsyn, R. H. Pain, G. B. Semisotnov, E. Zerovnik, and O. I. Razgulyaev. Evidence for a molten globule state as a general intermediate in protein folding. FEBS Lett. 262:20-24 (1990).
O. B. Ptitsyn and V. N. Uversky. The molten globule is a third thermodynamical state of protein molecules. FEBS Lett. 341:15-18 (1994).
M. Vincent. I. M. Li de la Sierra, M. N. Berberan-Santos, A. Diaz, M. Diaz, G. Padron, and J. Gallay. Time-resolved fluorescence study of human recombinant interferon a2. Association state of the protein, spatial proximity of the two tryptophan residues. Eur. J. Biochem. 210:953-962 (1992).
I. V. Dudich, E. I. Dudich, D. P. Kulevatskii, and V. P. Zav'yalov. Fluorescence polarization, circular dichroism and differential microcalorimetry study of structural features of human recombinant leukocyte interferon A. Mol. Biol. 25:1061-1070 (1991).
S. I. Borukhov and A. Y. Strongin. The intrinsic fluorescence of the recombinant human leukocyte interferon-αA and fibroblast interferon β1. Biochem. Biophys. Res. Commun. 169:282-288 (1990).
J. M. Davis, M. A. Narachi, H. L. Levine, N. K. Alton, and T. Arakawa. Conformation and stability of two recombinant human interferon-a analogs. Int. J. Pept. Prot. Res. 29:685-691 (1987).
M. Haria and P. Benfield. Interferon-α-2a: A review of its pharmacological properties and therapeutic use in the management of viral hepatitis. Drugs 50:873-896 (1995).
S. Baron, D. H. Coppenhaver, F. Dianzani, W. R. Fleischmann, Jr., T. K. Hughes, D. W. Niesel, G. J. Stanton, and S. K. Tyring. Introduction to the interferon system. In S. Baron, D. H. Coppenhaver, F. Dianzani, W. R. Fleischmann, Jr., T. K. Hughes, D. W. Niesel, G. J. Stanton, and S. K. Tyring (eds.), Interferon: Principles and Medical Applications, University of Texas Medical Branch, Galveston, Texas, 1992, pp. 1-15.
W. Klaus, B. Gsell, A. M. Labhardt, B. Wipf, and H. Senn. The three-dimensional high resolution structure of human interferon α-2a determined by heteronuclear NMR spectroscopy in solution. J. Mol. Biol. 274:661-675 (1997).
D. H. Tallmadge, J. S. Huebner, and R. F. Borkman. Acrylamide quenching of tryptophan photochemistry and photophysics. Photochem. Photobiol. 49:381-386 (1989).
J. R. Lakowicz. Principles of Fluorescence Spectroscopy, Plenum, New York, 1982.
M. R. Eftink and C. A. Ghiron. Fluorescence quenching studies with proteins. Anal. Biochem. 114:199-227 (1981).
M. R. Eftink and C. A. Ghiron. Dynamics of a protein matrix revealed by fluorescence quenching. Proc. Natl. Acad. Sci. USA 72:3290-3294 (1975).
S. S. Lehrer. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry 10:3254-3263 (1971).
M. R. Eftink. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys. J. 66:482-501 (1994).
Software downloaded from website http://www2.umdnj.edu/cdrwjweb.
N. Sreerama and R. W. Woody. Protein secondary structure from circular dichroism spectroscopy. Combining variable selection principle and cluster analysis with neural network, ridge regression and self-consistent methods. J. Mol. Biol. 242:497-507 (1994).
E. H. Strickland. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit. Rev. Biochem. 2:113-175 (1974).
R. H. I. Pain (ed.), Mechanisms of Protein Folding, Oxford University Press, Oxford, 2000.
M. G. Mulkerrin and R. Wetzel. pH dependence of the reversible and irreversible thermal denaturation of γ interferons. Biochemistry 28:6556-6561 (1989).
R. L. Remmele, Jr., N. C. Nightlinger, S. Srinivasan, and W. R. Gombotz. Interleukin-1 receptor (IL-1R) liquid formulation development using differential scanning calorimetry. Pharm. Res. 15:200-209 (1998).
R. L. Remmele, Jr., S. D. Bhat, D. H. Phan, and W. R. Gombotz. Minimization of recombinant human Flt3 ligand aggregation at the Tm plateau: A matter of thermal reversibility. Biochemistry 38:5241-5247 (1999).
A. Braun. and J. Alsenz. Development and use of enzyme-linked immunosorbent assays (ELISA) for the detection of protein aggregates in interferon alpha (IFN-α) formulations. Pharm. Res. 14:1394-1400 (1997).
G. Gunter, S. Del Terzo, and S. K. Kumar. Stabilized interferon alpha solutions, US Patent 5,762,923. (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V.K., Kalonia, D.S. Temperature- and pH-Induced Multiple Partially Unfolded States of Recombinant Human Interferon-α2a: Possible Implications in Protein Stability. Pharm Res 20, 1721–1729 (2003). https://doi.org/10.1023/B:PHAM.0000003367.62900.0f
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000003367.62900.0f