Abstract
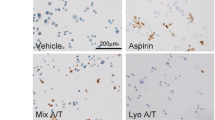
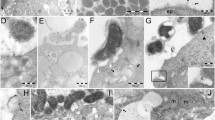
This study was undertaken to define the role that apoptosis may play in inducing cellular injury and death in gastric mucosa exposed to aspirin. Apoptosis was characterized by DNA gel electrophoresis, terminal deoxynucleotidyl transferase dUTP nick-end labeling assay, and DNA–histone-associated complex formation. A human gastric cell line (AGS) was exposed to physiologic concentrations (3 to 50 mM) of aspirin. Both time- and concentration-dependent effects on apoptosis were noted, which were effectively prevented by the caspase inhibitor z-VAD-fmk. Accordingly, the role of caspases in aspirin-induced apoptosis was also evaluated. Early activation of caspase-8 and caspase-9 was demonstrated, indicating a role for both receptor and mitochondrial pathways, respectively, in the apoptotic process. Corresponding activation of effector caspases-3, -6, and -7 was also evident, as was cleavage of PARP. We conclude that physiologically relevant concentrations of aspirin induces apoptosis in human gastric cells through a caspase-mediated mechanism.
Similar content being viewed by others
REFERENCES
Harris ED Jr: Rheumatoid arthritis: Pathophysiology and implications for therapy. N Engl J Med 322:1277–1289, 1990
Wipf JE, Deyo RA: Low back pain. Med Clin North Am 79:231–246, 1995
Hawkey CJ: Non-steroidal anti-inflammatory drug gastropathy. Gastroenterology 119:521–535, 2000
Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI: Gastrointestinal damage associated with the use of non-steroidal anti-inflammatory drugs. N Eng J Med 327:749–754, 1992
Wolfe MM, Lichtenstien DR, Singh G: Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. N Eng J Med 340:1888–1999, 1999
Rich M, Scheiman JM: Nonsteroidal anti-inflammatory drug gastropathy at the newmillennium: Mechanisms and prevention. Semin Arth Rheum 30:167–179, 2000
Miller TA, Smith GS, Baretto JC: Gastroduodenal defence: role of epithelial factors. In Immunopharmacology of Epithelial Barriers. R Goldie (ed). London, Academic Press, 1994, pp 197–211
Scheiman JM: NSAIDs, gastrointestinal injury and cytoprotection. Gastroenterol Clin North Am 25:279–298, 1996
Wallace JL: Mechanisms of non-steroidal anti-inflammatory drug (NSAID)-induced gastrointestinal damage: Potential for development of gastrointestinal tract safe NSAIDs. Can J Physiol Pharmacol 72(12):1493–1498, 1994
La Vecchia C, Negri E, Franceschi S, Conti E, Montella M, Giacossa A, Falcini A, Decarli A: Aspirin and colorectal cancer. Br J Cancer 76:675–677, 1997
Rosenberg L, Louik C, Shapiro S: Non-steroidal anti-inflammatory drug use and reduced risk of large bowel cancer. Cancer 82:2326–2333, 1998
Collet JP, Sharpe C, Belzile E, Boirin JF, Hanley J, Abenhaim L: Colorectal cancer prevention by non-steroidal anti-inflammatory drugs: Effects of dosage and timing. Br J Cancer 81:62–68, 1999
Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B: Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol 55:53–64, 1998
Shiff SJ, Koutsos MI, Qiao L, Rigas B: Non-steroidal anti-inflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: Effects on cell cycle and apoptosis. Exp Cell Res 222:179–188, 1996
Castano E, Dalmau M, Barragan M, Pueyo G, Bartrons R, Gil J: Aspirin induces cell death and caspase-dependent phosphatidylserine externalization in HT-29 human colon adenocarcinoma cells. Br J Cancer 81(2):294–299, 1999
Zhou XM, Wong BC, Fan XM, Zhang HB, Lin MC, Kung HF, Fan DM, Lam SK: Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis 22(9):1393–1397, 2001
Zhu GH, Wong BC, Slosberg ED, Eggo MC, Ching CK, Yuen ST, Lai KC, Weinstein B, Lam SK: Overexpression of protein kinase C-β1 isoenzyme suppresses indomethacin-induced apoptosis in gastric epithelial cells. Gastroenterology 18:507–514, 2000
Zhu GH, Wong BC, Eggo MC, Ching CK, Yuen ST, Chan EY, Lai KC, Lam SK: Non-steroidal anti-inflammatory drug-induced apoptosis in gastric cancer cells blocked by protein kinase C activation through inhibition of c-myc. Br J Cancer 79(3–4):393–400, 1999
Zhu GH, Wong BC, Ching CK, Lai KC, Lam SK: Differential apoptosis by indomethacin in gastric epithelial cells through the constitutive expression of wild-type p53 and/or up-regulation of c-myc. Biochem Pharmacol 58:193–200, 1999
Fax XG, Kelleher D, Fan XJ, Xia HX, Keeling PWN: Heliobacter pylori increases proliferation of gastric epithelial cells. Gut 37:19–22, 1996
Nilius M, Bode G, Buchler M, Malfertheiner P: Adhesion of Heliobacter pylori and Escherichia coli to human and bovine surface mucus cells in vitro. Eur J Clin Invest 24:454–459, 1994
Sharma SA, Tummuru MK, Miller GG and Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Heliobacter pylori stimulation in vitro. Infect Immun 63:1681–1687, 1995
Kokoska ER, Smith GS, Rieckenberg CL, Deshpande Y, Banan A, Miller, TA: Adaptive cytoprotection against deoxycholate-induced injury in human gastric cells in vitro: Is there a role for endogenous prostaglandins? Dig Dis Sci 43:806–815, 1998
Kokoska ER, Smith GS, Wolff AB, Deshpande Y, Rieckenberg, CL, Banan A, Miller TA: Role of calcium in adaptive cytoprotection and cell injury induced by deoxycholate in human gastric cells. Am J Physiol 275:322–330, 1998
Burgoyne LA, Hewish DR, Mobbs J: Mammalian chromatin substructure studies with the calcium-magnesium endonuclease and two-dimensional polyacrylamide gel elecrophoresis. Biochem J 143:67–72, 1974
Oberhammer F, Wilson JW, Dire C, Morris I D, Hickmann JA, Wakeling AE, Walker, PR, Sikopska M: Apoptotic death in epithelial cells: Cleavage of DNA to 300 and/or 50kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12:3679–3684, 1993
Facchinetti A, Tessarollo L, Mazzocchi M, Kingston R, Collavo D, Biasi G: An improved method for the detection of DNA fragmentation. J Immunol Methods 136:151–156, 1991
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976
Davenport HW: Fluid produced by gastric mucosa during damage by acetic and salicylic acids. Gastroenterology 50:487–499, 1966
Davenport HW: Damage to gastric mucosa: Effects of salicylates and stimulation. Gastroenterology 49:189–196, 1965
Davenport HW: Salicylate damage to the gastric mucosal barrier. N Engl J Med 276:1307–1312, 1967
Davenport HW. Why the stomach does not digest itself. Scientific American 226(1):87–93, 1972
Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3,-7 and-8. J Biol Chem 272:25719–25723, 1997
Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G. Mitochondrial release of caspase-2 and-9 during the apoptotic process. J Exp Med 189:381–394, 1999
Nunez G, Benedict MA, Hu Y, Inohara N: Caspases: The proteases of the apoptotic pathway. Oncogene 17(25):3237–3245, 1998
Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M: Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3 resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274:22932–22940, 1999
Decker P, Isenberg D, Muller S: Inhibition of caspase-3 mediated poly (ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis. J Biol Chem 275:9043–9046, 2000
Fiorucci S: NO-releasing NSAIDs are caspase inhibitors. Trends in immunology 22:232–235, 2001
Dimmeler S, Haendeler J, Nehls M, Zeiher AM: Suppression of apoptosis by nitric oxide via inhibition of interleukin-1 beta-converting enzyme (ICE)-like and cysteine protease protein (CPR)-32-like proteases. J Exp Med 185(4):601–607, 1997
Chan TA, Morin PJ, Vogelstein B, Kinzler KW: Mechanisms underlying non-steroidal anti-inflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA 95(2):681–686, 1998
Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr.: Aspirin use and risk of fatal cancer. Cancer Res 53(6):1322–1327, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Power, J.J., Dennis, M.S., Redlak, M.J. et al. Aspirin-Induced Mucosal Cell Death in Human Gastric Cells: Evidence Supporting an Apoptotic Mechanism. Dig Dis Sci 49, 1518–1525 (2004). https://doi.org/10.1023/B:DDAS.0000042258.41480.30
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000042258.41480.30