Abstract
Plant neocentromeres are large heterochromatic domains that associate with microtubules and move rapidly poleward during meiotic cell division. In maize, neocentromeres are part of a process that leads to the preferential recovery (meiotic drive) of knobs in progeny. These 'classical' plant neocentromeres differ from animal neocentromeres by their morphology, inability to mediate sister chromatid cohesion, and their rates of movement on the spindle. We provide a comprehensive review of classical neocentromeres with emphasis on their origin and mechanisms of motility. The data support the view that most, if not all, classical neocentromeres are the outcome of selection by meiotic drive. In addition, we compare and contrast neocentromere-mediated meiotic drive with a recently proposed meiotic drive model for centromere evolution.
Similar content being viewed by others
References
Ananiev EV, Phillips RL, Rines HW (1998a) A knob-associated tandem repeat in maize capable of forming fold-back DNA segments: Are chromosome knobs megatransposons? Proc Natl Acad Sci USA 95: 10785–10790.
Ananiev EV, Phillips RL, Rines HW (1998b) Complex structure of knob DNA on maize chromosome 9: Retrotransposon invasion into heterochromatin. Genetics 149: 2025–2037.
Ardlie KG (1998) Putting the brake on drive: meiotic drive of t haplotypes in natural populations of mice. Trends Genet 14: 189–193.
Bajer A (1958) Cine-micrographic studies on chromosome movements in b-irradiated cells. Chromosoma 9.
Bajer A (1969) Effect of UV microbeam irradiation on chromosome movements and spindle fine structure (with film demonstration). Biophys J 9: A151.
Bajer A, Mole-Bajer J (1963) Cine-analysis of some aspects of mitosis in endosperm. In Rose GG, ed. Cinemicrography in cell biology. New York: Academic Press, pp 357–409.
Bloom K (1993) The centromere frontier: kinetochore components, microtubule-based motility, and the CEN-value paradox. Cell 73: 621–624.
Bosemark NO (1956) On accessory chromosomes in Festuca pratensis. IV. Cytology and inheritance of small and large accessory chromosomes. Hereditas 42: 235–260.
Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B (1999) Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol 146: 1012.
Buckler ESI, Phelps-Durr TL, Buckler CSK, Dawe RK, Doebley JF, Holtsford TP (1999) Meiotic drive of chromosomal knobs reshaped the maize genome. Genetics 153: 415–426.
Chan A, Cande WZ (1998) Maize meiotic spindles assemble caround chromatin and do not require paired chromosomes. J Cell Sci 111: 3507–3515.
Charlesworth B, Hartl DL (1978) Population dynamics of the segregation distorter polymorphism of Drosophila melanogaster. Genetics 89: 171–192.
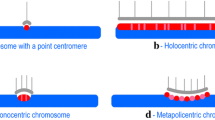
Choo KHA (2001) Domain organization at the centromere and neocentromere. Dev Cell 1: 165–177.
Church K, Nicklas RB, Lin HP (1986) Micro-manipulated bivalents can triggger mini-spindle formation in Drosophila melanogaster spermatocyte cytplasm. J Cell Biol 103: 2765–2733.
Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112: 407–421.
Compton DA (1998) Focusing on spindle poles. J Cell Sci 111: 1477–1481.
Czabab BB, Forer A, Bajer AS (1993) Ultraviolet microbeam irradiation of chromosomal spindle fibers in Haemanthus katherinae endosperm. J Cell Sci 105: 571–578.
Dawe RK, Cande WZ (1996) Induction of centromeric activity in maize by suppressor of meiotic drive 1. Proc Natl Acad Sci USA 93: 8512–8517.
Dawe RK, Reed L, YuH-G, Muszynski MG, Hiatt EN (1999) A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore. Plant Cell 11: 1227–1238.
Dennis ES, Peacock WJ (1984) Knob heterochromatin homology in maize and its relatives. J Mol Evol 20: 341–350.
Desai A, Maddox PS, Mitchison TJ, Salmon ED (1998) Anaphase A chromosome movement and poleward microtubule flux occur and similar rates in Xenopus extract spindles. J Cell Biol 141: 703–714.
Dimitrov B, Georgieva V (1994) Comparative analysis of sister-chromatid exchanges in plant and human chromosomes. Mut Res 304: 187–192.
Doolittle WF, Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603.
duSart D, Cancilla MR, Earle E et al. (1997) A functional neo-centromere formed through activation of a latent human centromere and consisting of non-alpha-satellite DNA. Nat Genet 16: 144–153.
Forer A, Spurck T, Pickett-Heaps JD, Wilson PJ (2003) Structure of kinetochore fibres in crane-fly spermatocytes after irradiation with an ultraviolet microbeam: neither microtubules nor actin filaments remain in the irradiated region. Cell Motil Cytoskeleton 56: 173–192.
Fuge H (1975) Anaphase transport of akinetochoric fragments in tipulid spermatocytes. Chromosoma 52: 149–158.
Funabiki H, Murray AW (2000) The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102: 411–412.
Goday C, Pimpinelli S (1989) Centromere organization in meiotic chromosomes of Parascaris univalens. Chromosoma 98: 160–166.
Goshima G, Vale RD (2003) The roles of microtubule-based otor proteins in mitosis: comprehensive RNAi analysis in he Drosophila S2 cell line. J Cell Biol 162: 1003–1016.
Gruss OJ, Wittmann M, Yokoyama H, et al. (2002) Chromosome-nduced microtubule assembly mediated by TPX2 is equired for spindle formation in HeLa cells. Nat Cell Biol 4: 71–879.
Haig D, Grafen A (1991) Genetic scrambling as a defence gainst meiotic drive. J Theor Biol 153: 531–558.
Ayman DL (1955) Centromeric behaviour of the univalents in wo Phalaris hybrids. Aust J Biol Sci 8: 241–253.
Hayward MD (1962) Genetic control of neocentric activity inrye. Heredity 17: 439–441.
Henikoff S, Ahmad K, Malik HS (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293: 1098–1102.
Hiatt EH (2000) Cytogenetic analysis of meiotic drive in maize. PhD thesis. University of Georgia, Athens.
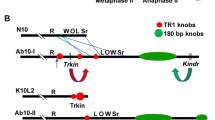
Hiatt EN, Dawe RK (2003a) The meiotic drive system on maize abnormal chromosome 10 contains few essential genes. Genetica 117: 67–76.
Hiatt EN, Dawe RK (2003b) Four loci on Abnormal chromosome 10 contribute to meiotic drive in maize. Genetics 164: 699–709.
Hiatt EN, Kentner EK, Dawe RK (2002) Independentlyregulated neocentromere activity of two classes of satellite sequences in maize. Plant Cell 14: 407–420.
Hilton H, Gaut BS (1998) Speciation and domestication in maize and its wild relatives: evidence from the globulin-1 gene. Genetics 150: 863–872.
Hirochika H, Sugimoto K, Otsuki Y, Sugawa T, Kanda M (1996) Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93: 7783–7788.
Hsu FC, Wang CJ, Chen CM, Hu HY, Chen CC (2003) Molecular characterization of a family of tandemly repeated DNA sequences, TR-1, in heterochromatic knobs of maize and its relatives. Genetics 164: 1087–1097.
Inoue´ S (1995) Foundations of confocal scanned imaging in light microscopy. In Handbook of biological confocal microscopy. New York: Plenum, pp 1–17.
Jain HK (1960) Induced neocentric activity in chromosome ends. Chromosoma 11: 310–312.
Jones GH (1969) Further correlations between Chiasmata and U-type exchanges in rye meiosis. Chromosoma 26: 105–118.
Karsenti E, Newport J, Kirschner M (1984) Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J Cell Biol 99: 47s–54s.
Kashkush K, Feldman M, Levy AA (2003) Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat Genet 33: 102–106.
Kato YTA (1976) Cytological studies of maize (Zea mays L.) and teosinte (Zea mexicana Shrader Kuntze) in relation to their origin and evolution. Mass Agric Exp Stn Bull 635:1–185.
Kattermann (1939) Ein neuer karyotyp bei Roggen. Chromosoma 1: 284–299.
Kavander T, Viinikka Y (1987) Neocentric activity in openpollinated cultivars of rye. Hereditas 107: 229–233.
Khodjakov A, Cole RW, Bajer AS, Rieder CL (1996) The force for poleward chromosome motion in Haemanthus cells acts along the length of the chromosome during metaphase but only at the kinetochore during anaphase. J Cell Biol 132:1093–1104.
Kikudome GY (1959) Studies on the phenomenon of preferential segregation in maize. Genetics 44: 815–831.
LaFountain JR, Oldenbourg R, Cole RW, Rieder CL (2001) Microtubule flux mediates poleward motion of acentric chromosome fragments during meiosis in insect spermatocytes. Mol Biol Cell 12: 4054–4056.
Lambert A-M (1993) Microtubule organizing centers in higher plants. Curr Opin Cell Biol 5: 116–122.
Lawrence CJ, Morris NR, Meagher RB, Dawe RK (2001) Dyneins have run their course in plant lineage. Traffic 2:362–363.
Lawrence CJ, Malmberg RL, Muszynski MG, Dawe RK (2002) Maximum likelihood methods reveal conservation of function among closely related kinesin families. J Mol Evol 54: 42–53.
Liang H, Wright WH, Cheng S, He W, Berns MW (1993) Micromanipulation of chromosomes in PTK2 cells using laser microsurgery (optical scalpel) in combination with laser-induced optical force (optical tweezers). Exp Cell Res 204: 110–120.
Lombillo VA, Stewart RJ, McIntosh JR (1995) Minus-end directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature 373: 161–163.
Longley AE (1937) Morphological characters of teosinte chromosomes. J Agric Res 54: 835–862.
Longley AE (1939) Knob positions on corn chromosomes. J Agric Res 59: 475–490.
Longley AE (1945) Abnormal segregation during megasporogenesis in maize. Genetics 30: 100–113.
Maddox P, Desai A, Oegema K, Mitchison MJ, Salmon ED (2002) Poleward microtubule flux is a major component of spindle dynamics and anaphase a in mitotic Drosophila embryos. Curr Biol 12: 1670–1674.
Malik HS, Henikoff S (2001) Adaptive evolution of Cid, a centromere-specific histone in Drosophila. Genetics 157: 293–1298.
Malik HS, Henikoff S (2002) Conflict begets complexity: the evolution of centromeres. Curr Opin Genet Dev 12: 711–718.
Manzanero S, Puertas MJ (2003) Rye terminal neocentromeres: characterization of the underlying DNA and chromatin structure. Chromosoma 111: 408–415.
Mazia D (1961) Mitosis and the physiology of cell division. In Brachet J, Mirsky AE, eds. The Cell, vol. III, New York: Academic Press, pp 77–422.
McAinsh AD, Tytell JD, Sorger PK (2003) Structure, function, and regulation of budding yeast kinetochores. Ann Rev Cell Dev Biol 19: 519–539.
McClintock B (1943) Maize genetics. Yearbook Carnegie Inst Wash 42: 148–152.
B. pseudolaevipes. Genetics 37: 8-25. Walters MS (1952b) A typical chromosome movement in meiotic anaphase of Bromus pitensis _ B. marginatus. Am J Bot 39: 619-625. Waters JC, Mitchison TJ, Rieder CL, Salmon ED (1996) The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell 7: 1547-1558. Williams BC, Murphy TD, Goldberg ML, Karpen GH (1998) Neocentromere activity of structurally acentric mini-chromosomes in Drosophila. Nat Genet 18: 30-37. Wood KW, Sakowicz R, Goldstein LSB, Cleveland DW (1997) CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91: 357-366. Wu C-I, Hammer MF (1990) Molecular evolution of ultraselfish genes of meiotic drvie systems. In Sclander RK, Whittam T, Clark A, eds. Evolution at the molecular level. Sunderland, Massachusetts: Sinauer. Yu H-G (2000) The maize kinetochore: composition, structure and roles in meiotic chromosome segregation. PhD thesis, University of Georgia, Athens. YuH-G, Hiatt EN, Chan A, Sweeney M, Dawe RK (1997) Neocentromere-mediated chromosome movement in maize. J Cell Biol 139: 831-840. YuH-G, Muszynski MG, Dawe RK (1999) The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J Cell Biol 145: 425-435. YuH-G, Hiatt EN, Dawe RK (2000) The plant kinetochore. Trends Plant Sci 5: 543-547. Zhong CX, Marshall JB, Topp C, et al. (2002) Centromeric retroelements and satellites interact with maize kinetochore protein CENH3. Plant Cell 14: 2825-2836. Zohary D (1955) Secondary centric activity in Lilium formosanum. Am Nat 89: 50-52. Maize meiotic drive 669
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dawe, R.K., Hiatt, E.N. Plant neocentromeres: fast, focused, and driven. Chromosome Res 12, 655–669 (2004). https://doi.org/10.1023/B:CHRO.0000036607.74671.db
Issue Date:
DOI: https://doi.org/10.1023/B:CHRO.0000036607.74671.db