Abstract
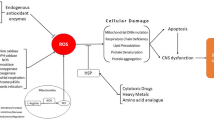
Hypotheses about the factors controlling the rate of brain aging are usually derived from 1) correlates of maximum life span across mammals or 2) investigations into the causes of age-related neuropathologies in humans. With regard to the former, the strong correlation between metabolic rate and longevity prompted a variety of free radical hypotheses of aging. There is also evidence that brain size affects life span independently of body metabolism rates. The second approach has led to a diverse array of pathogenic mechanisms and, importantly for the development of general hypotheses, the discovery of animal analogues. The present paper discusses the possibility that age-associated lysosomal dysfunction constitutes a generalized mammalian phenomenon that accounts for specific features of the aged human brain. Immunocytochemical studies using rats and dogs have identified lysosomal changes that begin early in adulthood and are most pronounced in brain areas known to be particularly vulnerable to age-related pathogenesis in humans. Experimentally induced lysosomal dysfunction in cultured brain slices from rats and mutant mice triggers a wide array of changes associated with the aged human brain, including meganeurites and intraneuronal tangles. Finally, there is evidence that at least some forms of proteolysis decrease with increasing brain size across the mammals. The above observations lead to the suggestion that the expansion of neuronal arborizations that occurred in conjunction with increases in brain size secondarily slowed both neuronal metabolism and protein turnover. These events could have served to reduce the rate at which lysosomes (and other organelles) fail.
Similar content being viewed by others
REFERENCES
Harman, D. 1972. The biological clock: The mitochondria? J. Am. Geriatr. Soc. 20:145-147.
Finch, C. E. 1990. Longevity, senescence, and the genome. Chicago, University of Chicago Press.
Sacher, G. A. 1959. Relation of lifespan to brain weight and body weight in mammals. in Wohstenholme, G. E. W. and O'Connor, M., (eds.), Collog Aging, CIBA Foundation, London.
Allman, I., McLaughlin, T., and Hakeem, A. 1993. Brain weight and lifespan in primate species. Proc. Natl. Acad. Sci. USA 90:118-122.
Braak, H. and Braak, E. 1991. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82:239-259.
Lynch, G., Larson, J., and Baudry, M. 1986. Proteases, neurostability, and brain aging: An hypothesis, in Crook, T., Bartus, R. Ferris, D. Garshon, N. (eds.), Treatment Development Strategies for Alzheimer's Disease, Mark Powley Assoc. (publisher) New York.
Kleiber, M. 1947. Body size and metabolic rate. Physiol. Rev. 27:511-541.
Heusner, A. A. 1982. Energy metabolism and body size: I. Is the 0.75 mass exponent of Kleiber's equation a statistical artifact? Respir. Physiol. 48:1-12.
Comfort, A. 1979. The Biology of Senescence, New York, Elservier.
Barja, G. 1998. Mitochondrial free radical production and aging in mammals and birds. Ann. N Y Acad. Sci. 854:224-238.
Economos, A. C. 1980. Taxonomic differences in the mammalian lifespan-body weight relationship and the problem of brain weight. Gerontology 26:90-98.
Hofman, M. A. 1983. Energy metabolism, brain size and longevity in mammals. Q. Rev. Biol. 58:495-512.
Guillozet, A. L. Weintraub, S., Mash, D. C., and Mesulam, M. M. 2003. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch. Neurol. 60:729-736.
Riley, K. P., Snowdon, D. A., and Markesbery, W. R. 2002. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: Findings from the Num Study. Ann. Neurol. 51:567-577.
Mitchell, T. W., Mufson, E. J., Schneider, J. A., Cochran, E. J., Nissanov, J., Han, I. Y., Bienias, J. L., Lee, V. M., Trojanowski, J. O., Bennett, D. A., and Arnold, S. E. 2002. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease. Ann. Neurol. 51:182-189.
Cummings, B. J., Satou, T., Head, E., Milgram, N. W., Cole, G. M., Savage, M. J., Podlisny, M. B., Selkoe, D. J., Siman, R., Greenberg, B. D., and Cotman, C. W. 1996. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: Evidence from cats and dogs. Neurobiol. Aging 17:653-659.
Papaioannou, N., Tooten, P. C., van Ederen, A. M., Bohl, J. R., Rofina, J., Tsangaris, T., Gruys, E. 2001. Immunohistochemical investigation of the brain of aged dogs: I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid 8:11-21.
Braak, H., Braak, E., and Strothjohann, M. 1994. Abnormally phosphorylated tau protein related to the formation of neurofibrillary tangles and neuropil threads in the cerebral cortex of sheep and goat. Neurosci. Lett. 171:1-4.
Schultz, C., Hubbard, G. B., Rub, U., Braak, E., and Braak, H. 2000. Age-related progression of tau pathology in brains of baboons. Neurobiol. Aging 21:905-912.
Gearing, M., Tigges, J., and Mori, H., and Mirra, S. S. 1997. Beta-amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol. Aging 18:139-146.
Braak, E., Braak, H., Strenge, H., and Muhtaroglu, A. U. 1980. Age-related alterations of the proximal axon segment in lamina IIIab-pyramidal cells of the human isocortex: A Golgi and fine structural study. J. Hirnforsch. 21:531-553.
Braak, H. 1979. Spindle-shaped appendages of IIIab-pyramids filled with lipofuscin: A striking pathological change of the senescent human isocortex. Acta Neuropathol. (Berl.) 46:197-202.
Nakayama, H., Kiatipattanasakul, W. Nakamura, S., Miyawaki, K., Kikuta, F., Uchida, K., Kuroki, K., Makifuchi, T., Yoshikawa, Y., and Doi, K. 2001. Fractal analysis of senile plaque observed in various animal species. Neurosci. Lett. 297:195-198.
Geula, C., Nagykery, N., and Wu, C. K. 2002. Amyloid-beta deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): Incidence and chemical composition. Acta Neuropathol. (Berl.) 103:48-58.
Sani, S., Traul, D., Klink, A., Niaraki, N., Gonzalo-Ruiz, A., Wu, C. K., and Geula, C. 2003. Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: Similarities to the human. Acta Neuropathol. (Berl.) 105:145-156.
Kimura, N., Nakamura, S., Goto, N., Narushima, E., Hara, I., Shichiri, S., Saitou, K., Nose, M., Hayashi, T., Kawamura, S., Yoshikawa, Y. 2001. Senile plaques in an aged western lowland gorilla. Exp. Anim. 50:77-81.
Cotman, C. W., Head, E., Muggenburg, B. A., Zicker, S., and Milgram, N. W. 2002. Brain aging in the canine: A diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol. Aging 23:809-818.
Head, E., McCleary, R., Hahn, F. F., Milgram, N. W., Cotman, C. W. 2000. Region-specific age at onset of beta-amyloid in dogs. Neurobiol. Aging 21:89-96.
Cummings, B. J., Su, J. H., Cotman, C. W., White, R., and Russell, M. J. 1993. Beta-amyloid accumulation in aged canine brain: A model of early plaque formation in Alzheimer's disease. Neurobiol. Aging 14:547-560.
Braak, H. and Braak, E. 1997. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 18:351-357.
Kitani, K., Ivy, G. O., and Shimasaki, H. 1995. Lipofuscin and ceroid pigments: State of the art. Gerontology 41(suppl 2):1-330.
Oenzil, F., Kishikawa, M., Mizuno, T., and Nakano, M. 1994. Age-related accumulation of lipofuscin in three different regions of rat brain. Mech. Ageing Dev. 76:157-163.
Nakanishi, H., Tominaga, K., Amano, T., Hirotsu, I., Inoue, T., and Yamamoto, K. 1994. Age-related changes in activities and localizations of cathepsins D, E, B, and L in the rat brain tissues. Exp. Neurol. 126:119-128.
Nakamura, Y., Takeda, M., Suzuki, H., Morita, H., Tada, K., Hariguchi, S., and Nishimura, T. 1989. Lysosome instability in aged rat brain. Neurosci. Lett. 97:215-220.
Kenessey, A., Banay-Schwartz, M., DeGuzman, T., and Lajtha, A. 1989. Increase in cathepsin D activity in rat brain in aging. J. Neurosci. Res. 23:454-456.
Nakamura, Y., Takeda, M., Suzuki, H., Morita, H., Tada, K., Hariguchi, S., and Nishimura, T. 1989. Age-dependent change in activities of lysosomal enzymes in rat brain. Mech. Ageing Dev. 50:215-225.
Nakanishi, H., Amano, T., Sastradipura, D. F., Yoshimine, Y., Tsukuba, T., Tanabe, K., Hirotsu, I., Ohono, T., and Yamamoto, K. 1997. Increased expression of cathepsins E and D in neurons of the aged rat brain and their colocalization with lipofuscin and carboxy-terminal fragments of Alzheimer amyloid precursor protein. J. Neurochem. 68:739-749.
Troncoso, J. C., Cataldo, A. M., Nixon, R. A., Barnett, J. L., Lee, M. K., Checler, F., Fowler, D. R., Smialek, J. E., Crain, B., Martin, I. J., and Kawas, C. H. 1998. Neuropathology of pre-clinical and clinical late-onset Alzheimer's disease. Ann. Neurol. 43:673-676.
Cataldo, A. M., Paskevich, P. A., Kominami, E., and Nixon, R. A. 1991. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 88:10998-11002.
Callahan, L. M., Vaules, W. A., and Coleman, P. D. 1999. Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 58:275-287.
Schwagerl, A. L., Mohan, P. S., Cataldo, A. M., Vonsattel, J. P., Kowall, N. W., and Nixon, R. A. 1995. Elevated levels of the endosomal-lysosomal proteinase cathepsin D in cerebrospinal fluid in Alzheimer disease. J. Neurochem. 64:443-446.
Bi, X., Yong, A. P., Zhou, J., Gall, C. M., and Lynch, G. 2000. Regionally selective changes in brain lysosomes occur in the transition from young adulthood to middle age in rats. Neuroscience 97:395-404.
Bi, X., Head, E., Cotman, C., and Lynch, G. 2003. Spatial patterns of mammalian brain aging: Distribution of cathepsin D immunore-active cell bodies and dystrophic dendrites in aging dogs resembles that in Alzheimer's disease. J. Comp. Neurol. 464:371-381.
Braak, H. 1980. Architectonics of the Human Telencephalic Cortex. Berlin, Springer-Verlag.
Ivy, G. O., Schottler, F., Wenzel, J., Baudry, M., and Lynch, G. 1984. Inhibitors of lysosomal enzymes: Accumulation of lipofuscin-like dense bodies in the brain. Science 226:985-987.
Ivy, G. O., Kitani, K., and Ihara, Y. 1989. Anomalous accumulation of tau and ubiquitin immunoreactivities in rat brain caused by protease inhibition and by normal aging: A clue to PHF pathogenesis? Brain Res. 498:360-365.
Bahr, B. A., Abai, B., Gall, C. M., Vanderklish, P. W., Hoffman, K. B., and Lynch, G. 1994. Induction of beta-amyloid-containing polypeptides in hippocampus: Evidence for a concomitant loss of synaptic proteins and interactions with an excitotoxin. Exp. Neurol. 129:81-94.
Bednarski, E., Ribak, C. E., and Lynch, G. 1997. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J. Neurosci. 17:4006-4021.
Bi, X., Zhou, J., and Lynch, G. 1999. Lysosomal protease inhibitors induce meganeurites and tangle-like structures in entorhinohippocampal regions vulnerable to Alzheimer's disease. Exp. Neurol. 158:312-327.
Yong, A. P., Bednarski, E., Gall, C. M., Lynch, G., and Ribak, C. E. 1999. Lysosomal dysfunction results in lamina-specific meganeurite formation but not apoptosis in frontal cortex. Exp. Neurol. 157:150-160.
Bi, X., Haque, T. S., Zhou, J., Skillman, A. G., Lin, B., Lee, C. E., Kuntz, I. D., Ellman, J. A., and Lynch, G. 2000. Novel cathepsin D inhibitors block the formation of hyperphosphorylated tau fragments in hippocampus. J. Neurochem. 74:1469-1477.
Bednarski, E. and Lynch, G. 1996. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J. Neurochem. 67:1846-1855.
Bi, X., Yong, A. P., Zhou, J., Ribak, C. E., and Lynch, G. 2001. Rapid induction of intraneuronal neurofibrillary tangles in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 98:8832-8837.
Tower, D. B. 1954. Structural and functional organization of mammalian cerebral cortex: The correlation of neuron density with brain size. J. Comp. Neurol. 101:19-51.
Changizi, M. A. 2001. Principles underlying mammalian neocortical scaling. Biol. Cybern. 84:207-215.
Bok, S. T. 1959. Histonomy of the Cerebral Cortex, Amsterdam, Elsevier.
Shariff, G. A. 1953. Cell counts in the primate cerebral cortex. J. Comp. Neurol. 98:381-400.
Baudry, M., Simonson, L., Dubrin, R., and Lynch, G. 1986. A comparative study of soluble calcium-dependent proteolytic activity in brain. J. Neurobiol. 17:15-28.
Abraham, W. C., Logan, B., Greenwood, J. M., and Dragunow, M. 2002. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 22:9626-9634.
Staubli, U. and Lynch, G. 1987. Stable hippocampal long-term potentiation elicited by 'theta' pattern stimulation. Brain Res. 435:227-234.
Staubli, U. and Lynch, G. 1990. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 513:113-118.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lynch, G., Bi, X. Lysosomes and Brain Aging in Mammals. Neurochem Res 28, 1725–1734 (2003). https://doi.org/10.1023/A:1026069223763
Issue Date:
DOI: https://doi.org/10.1023/A:1026069223763