Abstract
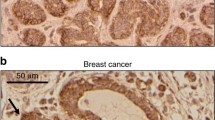
Seprase is a cell surface serine protease that is expressed to high levels by infiltrating ductal carcinomas of the breast but its function in malignancy is unknown. MDA-MB-435 (WT435) and MDA-MB-436 (WT436) human breast cancer cells express high levels of seprase as do the carcinoma cells in tumors of human breast cancer patients. To investigate its role in the pathobiology of breast cancer, seprase was specifically reduced in WT436 and WT435 cells by expression of antisense seprase cDNA. Decreased expression of seprase was confirmed in the antisense transfectants by zymography, immunoblotting, and fluorescence-activated cell sorting of cells labeled with antibody to seprase. Control-transfectants continued to express high levels of seprase. Seprase-deficient cells growing on type I collagen gels reveal a markedly different morphology than the parental or control-transfected cells that express high levels of seprase. The seprase-deficient cells grow in islands and aggregates of tightly attached cells while cells with high seprase expression grow as groups of separate individual cells. Interestingly, the aggregated growth of the seprase-deficient cells was not correlated with increased expression of E-cadherin. Seprase-deficient breast cancer cells also exhibit altered growth properties. Seprase-deficient cells and those with high seprase levels proliferate in serum-containing media. However, in serum-free medium seprase-deficient cells proliferate much more slowly than their seprase-expressing counterparts. These findings indicate that seprase promotes the aberrant growth of breast cancer cells by reducing their dependence on exogenous growth factors. Seprase may contribute to the pathogenesis of breast cancer by promoting growth of the primary tumor and by facilitating the growth of breast cancer cells in metastases at other sites of the body.
Similar content being viewed by others
References
Kelly T, Kechelava S, Rozypal TL et al. Seprase, a membrane—bound protease, is overexpressed by invasive and microinvasive ductal carcinoma cells of human breast cancers. Modern Pathol 1998; 11: 855–63.
Kelly T. Evaluation of seprase activity. Clin Exp Metastasis 1999; 17: 57–62.
Aoyama A. and Chen W—T. A 170—kDa membrane—bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc Natl Acad Sci USA 1990; 87: 8296–300.
Monsky WL, Lin C—Y, Aoyama A et al. A potential marker protease of invasiveness, seprase, is localized to invadopodia of human malignant melanoma cells. Cancer Res 1994; 54: 5702–10.
Kelly T, Mueller SC, Yeh Y et al. Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. J Cell Physiol 1994; 158: 299–308.
Rettig WJ, Garin—Chesa P, Beresford HR et al. Cell—surface glycoproteins of human sarcomas differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA 1988; 85: p. 3110–4.
Garin—Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA 1990; 87: 7235–9.
Chen W—T, Kelly T, Ghersi G. DPPIV, seprase and related serine peptidases in multiple cellular functions. In Zucker S, Chen W—T (ed): Cell Surface Proteases. San Diego, California: Academic Press 2003.
Park JE, Lenter MC, Zimmermann RN et al. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 1999; 274: 36505–12.
Levy MT, McCaughan GW, Abbott CA et al. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 1999; 29: 1768–78.
Pineiro—Sanchez M, Goldstein LA, Dodt J et al. Identification of the 170 kDa melanoma membrane—bound gelatinase (seprase) as a serine integral membrane protease. J Biol Chem 1997; 272: 7595–7601.
Scanlan MJ, Raj BK, Calvo B et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA 1994; 91: 5657–61.
Goldstein LA, Ghersi G, Pineiro—Sanchez ML et al. Molecular cloning of seprase: A serine integral membrane protease from human melanoma. Biochim Biophys Acta 1997; 1361: 11–9.
Niedermeyer J, Kriz M, Hilberg F et al. Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol 2000; 20: 1089–94.
Nakahara H, Nomizu M, Akiyama SK et al. A mechanism for regulation of melanoma invasion. J Biol Chem 1996; 271: 27221–4.
Chen W—T. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool 1989; 251: 167–85.
Chen W—T. Membrane proteases: Roles in tissue remodeling and tumour invasion. Curr Opin Cell Biol 1992; 4: 802–9.
Chen W—T, Lee CC, Goldstein L et al. Membrane proteases as potential diagnostic and therapeutic targets for breast malignancy. Breast Cancer Res Treat 1994; 31: 217–26.
Coopman PJ, Thomas DM, Gehlsen KR et al. Integrin α3β1 participates in the phagocytosis of extracellular matrix molecules by human breast cancer cells. Mol Biol Cell 1996; 7: 1789–804.
Kelly T, Yan Y, Osborne RL et al. Proteolysis of extracellular matrix by invadopodia facilitates human breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin ExpMetastasis 1998; 16: 501–12.
Monsky WL, Kelly T, Lin CY et al. Binding and localization of M r 72 000 matrix metalloproteinase at cell surface invadopodia. Cancer Res 1993; 53: 3159–64.
Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press 1989.
Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 1980; 102: 196–202.
Pumphrey CY, Theus AM, Li S et al. Neoglycans, carbodiimide—modified glycosaminoglycans: A new class of anticancer agents that inhibit cancer cell proliferation and induce apoptosis. Cancer Res 2002; 62: 3722–8.
Pieters R, Huismans DR, Leyva A et al. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Lett 1988; 41: 323–32.
Goldstein LA, Chen WT. Identification of an alternatively spliced seprase mRNA that encodes a novel intracellular isoform. J Biol Chem 2000; 275: 2554–9.
Dong J, Opresko LK, Dempsey PJ et al. Metalloprotease—mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc Natl Acad Sci USA 1999; 96: 6235–40.
Kahn ML, Hammes SR, Botka C. et al. Gene and locus structure and chromosomal localization of the protease—activated receptor gene family. J Biol Chem 1998; 273: 23290–6.
Vu TK, Hung DT, Wheaton VI et al. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991; 64: 1057–68.
Ishihara H, Connolly AJ, Zeng D et al. Protease—activated receptor 3 is a second thrombin receptor in humans. Nature 1997; 386: 502–6.
Even—Ram SC, Maoz M, Pokroy E et al. Tumor cell invasion is promoted by activation of protease activated receptor–1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem 2001; 276: 10952–62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goodman, J.D., Rozypal, T.L. & Kelly, T. Seprase, a membrane-bound protease, alleviates the serum growth requirement of human breast cancer cells. Clin Exp Metastasis 20, 459–470 (2003). https://doi.org/10.1023/A:1025493605850
Issue Date:
DOI: https://doi.org/10.1023/A:1025493605850