Abstract
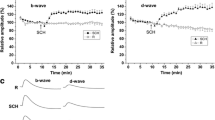
Purpose: Compare the effects of Nembutal, a barbiturate, and Telazol, a dissociative anesthetic, on photoreceptor and post-receptoral retinal responses. Methods: Dark adapted infant and adult albino rats were anesthetized with intraperitoneal injection of Nembutal or Telazol. ERG responses to full-field stimuli were recorded over a 6–8 log unit range from dim intensities at which the scotopic threshold response (STR) was observed to those that saturate the a-wave. The rod photoresponse, b-wave, oscillatory potentials (OPs), and STR parameters of rats in the Nembutal and Telazol groups were compared. Results: For both infants and adults, the saturated amplitudes of the photoresponse and the b-wave were larger in Telazol than Nembutal rats, but sensitivity of the photoresponse did not differ significantly between Telazol and Nembutal rats. The STR was seen only in the Telazol responses of adults. There was little differential effect of the two agents on the OPs. Conclusions: Photoreceptor and postreceptoral responses recorded under Nembutal and Telazol anesthesia differ significantly. These results may inform selection of anesthetic for studies of animal models.
Similar content being viewed by others
References
Jamison JA, Bush RA, Lei B, Sieving PA. Characterization of the rod photoresponse isolated from the dark adapted primate ERG. Vis Neurosci 2001; 18: 445–55.
Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 1994; 35: 2948–61.
Breton ME, Schueller A, Lamb TD, Pugh EN Jr. Analysis of ERG a-wave amplification and kinetics in terms of the Gprotein cascade of phototransduction. Invest Ophthalmol Vis Sci 1994; 35: 295–309.
Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci 1992; 8: 107–26.
Hood D, Birch D. Beta wave of the scotopic (rod) electroretinogram as ameasure of the activity of human on-bipolar cells. J Opt Soc Am A 1996; 13: 623–33.
Aleman T, LaVail MM, Montemayor R et al. Augmented rod bipolar cell function in partial receptor loss: an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res 2001; 41: 2779–97.
Bush RA, Sieving PA. A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 1994; 35: 635–45.
Wurziger K, Lichtenberger T, Hanitzsch R. On-bipolar cells and depolarising third order neurons as the origin of the ERG b-wave in the RCS rat. Vision Res 2001; 41: 1091–101.
Robson J, Frishman L. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 1995; 12: 837–50.
Robson JG, Frishman LJ. Photoreceptor and bipolar cell contributions to the cat electroretinogram: A kinetic model for the early part of the flash response. J Opt Soc Am A 1996; 13: 613–22.
Sieving PA, Frishman LJ, Steinberg RH. Scotopic threshold response of proximal retina in the cat. J Physiol 1986; 56: 1049–61.
Pugh EN Jr, Falsini B, Lyubarsky AL. The origin of the major rod and cone driven components of the rodent electroretinogram and the effect of age and light rearing history on the magnitude of these components. In: Williams TP, Thistle AB, eds. Photostasis and Related Phenomena. New York: Plenum Press, 1998: 93–128.
Naarendorp FA, Sato Y, Cajdric A, Hubbard NP. Absolute and relative sensitivity of the scotopic system of the rat: Electroretinography and behavior. Vis Neurosci 2001; 18: 641–56.
Reynaud X, Hansen RM, Fulton AB. Effect of prior oxygen exposure on the electroretinographic responses of infant rats. Invest Ophthalmol Vis Sci 1995; 36: 2071–9.
Penn JS, Thum LA, Rhem MN, Dell SJ. Effects of oxygen rearing on the electrortinogram and GFA-protein in the rat. Invest Ophthalmol Vis Sci 1988; 29: 1623–30.
Lachapelle P, Dembinska O, Rojas LM, Benoit J, Almazan G, Chemtob S. Persistent functional and structural retinal abnormalities in newborn rats exposed to hyperoxia. Can J Physiol Pharmacol 1999; 77: 48–55.
Kapousta-Bruneau N. Effects of sodium pentobarbital on the components of the electroretinogram in the isolated rat retina. Vision Res 1999; 39: 3498–512.
Lin HC, Thurmon JC, Benson GJ, Tranquilli WJ. Telazol – a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther 1993; 16: 383–418.
Fulton AB, Hansen RM, Findl O. The development of the rod photoresponse from dark adapted rats. Invest Ophthalmol Vis Sci 1995; 36: 1038–45.
Findl O, Hansen RM, Fulton AB. The effects of acetazolamide on ERG responses in rats. Invest Ophthalmol Vis Sci 1995; 36: 1019–26.
Lamb TD, Pugh EN Jr. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 1992; 449: 719–58.
Pugh EN Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1993; 1141: 111–49.
Peachey NS, Alexander KR, Fishman GA. The luminanceresponse function of the dark-adapted human electroretinogram. Vision Res 1989; 29: 263–70.
Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Ret Eye Res 1998; 17: 485–521.
Lyubarsky AL, Pugh EN Jr. Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci 1996; 16: 563–71.
Frishman LJ, Steinberg RH. Light evoked increases in K+ in proximal portion of dark adapted cat retina. J Neurophysiol 1989; 61: 1233–43.
Vaegan, Millar TJ. Effect of kainic acid and NMDA on the pattern electroretinogram, the scotopic threshold response, the oscillatory potentials and the electroretinogram in the urethane anaesthetized cat. Vision Res 1994; 34: 1111–25.
Wachtmeister L. Basic research and clinical aspects of the oscillatory potentials of the electroretinogram. Doc Ophthalmol 1987; 66: 187–94.
Ogden TE. The oscillatory waves of the primate electroretinogram. Vision Res 1973; 13: 1059–74.
King-Smith P, Loffing DH, James R. Rod and cone ERG's and their oscillatory potentials. Invest Ophthalmol Vis Sci 1986; 27: 270–3.
Lim DM-K. Neurotransmitters in the vertebrate retina. Invest Ophthalmol Vis Sci 1997; 38: 553–6.
Ito T, Suzuki T, Wellman SE, Ho IK. Pharmacology of barbiturate tolerance/dependence: GABA A receptors and molecular aspects. Life Sci 1996; 59: 169–95.
Wood PL, Rao TS. A review of in vivo modulation of cerebellar cGMP by excitatory amino acid receptros:role of NMDA, quisqualate and kainate subtypes. Prog Neuro-Psychopharmacol Biol Psychiatry 1991; 15: 229–35.
Robson JG, Frishman LJ. Dissecting the dark adapted electroretinogram. Docum Ophthalmol 1999; 95: 187–215.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaudhary, V., Hansen, R., Lindgren, H. et al. Effects of telazol and nembutal on retinal responses. Doc Ophthalmol 107, 45–51 (2003). https://doi.org/10.1023/A:1024444113700
Issue Date:
DOI: https://doi.org/10.1023/A:1024444113700