Abstract
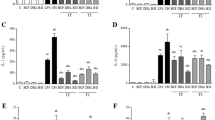
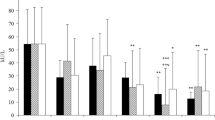
Phagocytic cells contain NADPH oxidase that they use for host defense by catalyzing the production of superoxide. Bacterial lipopolysaccharide (LPS) has been found to stimulate NADPH oxidase in mobile and sessile macrophages and microglia. It also evokes fever in homeothermic animals and men, a reaction mediated by central nervous system (CNS) activities. The purpose of the present study was to determine whether reactive oxygen species are involved in LPS-induced fever. In rabbits we found that plasma hydroperoxide levels increased and catalase activity decreased 15 min after LPS injection and that fever started with a similar latency, while plasma levels of tumor necrosis factor-α (TNFα) increased 30 min after the injection. Treating rabbits with methylene blue or aspirin did not affect TNFα secretion but prevented the LPS-induced rise of hydroperoxides and the inactivation of catalase, abolishing fever. Incubation of human blood with nitroblue tetrazolium and LPS increased the number of formazan-positive neutrophils from 10 ± 5 to 52 ± 9%. Adding LPS to blood preincubated with either methylene blue, α-lipoic acid, or aspirin respectively decreased the number of formazan-positive neutrophils to 0.9 ± 0.8, 0.8 ± 0.9, or 2.0 ± 0.9%, disclosing the antioxidant capacity of these drugs. Systemic application of 80 mg/kg α-lipoic acid elicited heat-loss reactions within 15 min and decreased core temperature by 2.2 ± 0.3°C within 2 h. α-Lipoic acid applied 45 min after LPS induced antipyresis within 15 min, and this antipyresis was associated with a decrease of elevated hydroperoxide levels and restoration of catalase activity. Our results show that fever is prevented when the production of reactive oxygen species is blocked and that an elevated body temperature returns to normal when oxygen radical production decreases. Estimation of plasma dihydrolipoic acid (DHLA) levels following injection of 80 mg/kg α-lipoic acid in afebrile and febrile rabbits revealed that this acid is converted into DHLA, which in afebrile rabbits increased the plasma DHLA concentration from 2.22 ± 0.26 μg/ml to peak values of 8.60 ± 2.28 μg/ml DHLA within 30 min and which in febrile rabbits increased it from 0.84 ± 0.22 μg/ml to peak values of 3.90 ± 0.94 μg/ml within 15 min. Methylene blue, aspirin, and α-lipoic acid, which all cross the blood–brain barrier, seem to act not only on peripheral tissues but also on the CNS. Brain structures that have been shown to sense oxidative stress are vicinal thiol groups attached to the NMDA subtype of glutamate receptor. Their reduction by thiol-reducing drugs like dithiothreitol or DHLA has been found to increase glutamate-mediated neuronal excitability, while the opposite effect has been observed after their oxidation. Because we found that systemic application of α-lipoic acid in the afebrile state elicits hypothermia and in the febrile state is antipyretic, we think this type of NMDA receptor is involved in thermoregulation and that oxidation of its thiol groups induces fever. It appears that temperature homeostasis can be maintained only if the redox homeostasis of the brain is guaranteed.
Similar content being viewed by others
References
Rossi F: The O2-forming NADPH oxidase of the phagocytes: Nature, mechanisms of activation and function. Biochim Biophys Acta 853: 65-89, 1986
Grisham MB, Everse J, Janssen HF: Endotoxemia and neutrophil activation in vivo. Am J Physiol 254: H1017-H1022, 1988
Fridovich I: The biology of oxygen radicals. Science 201: 875-880, 1978
Babior BM: NADPH oxidase: An update. Blood 93: 1464-1476, 1999
Cadenas E: Biochemistry of oxygen toxicity. Annu Rev Biochem 58: 79-110, 1989
Yusa T, Beckman JS, Crapo JD, Freeman BA: Hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol 63: 353-358, 1987
Toth LA, Blatteis CM: Adaptation to the microbial environment. In: M.J. Fregly, C.M. Blatteis (eds). Handbook of Physiology, Section 4, Environmental Physiology, Vol. I. Oxford University Press, New York, 1996, pp 1489-1519
Milton AS, Wendlandt S: Effects on body temperatures of prostaglandins of the A, E and F series on injection into the third ventricle of unanaesthetized cats and rabbits. J Physiol 218: 325-336, 1971
Brenman JE, Bredt DS: Synaptic signaling by nitric oxide. Curr Opin Neurobiol 7: 374-378, 1997
Lipton SA, Chol YB, Pan ZH, Lei SZ, Chen HSV, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS: A redox-based mechanism for the neuro-protective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364: 626-632, 1993
Aizenman E, Lipton SA, Loring RH: Selective modulation of NMDA responses by reduction and oxidation. Neuron 2: 1257-1263, 1989
Tang L-H, Aizenman E: Allosteric modulation of the NMDA receptor by dihydrolipoic and lipoic acid in rat cortical neurons in vitro. Neuron 11: 857-863, 1993
Canini F, Bréjot T, D'Aléo P, Mercier S, Bourdon L: NMDA receptors are involved in dithiothreitol-induced hypothermia. Eur J Pharmacol 426: 179-183, 2001
Riedel W, Maulik G: Fever: An integrated response of the central nervous system to oxidative stress. Mol Cell Biochem 196: 125-132, 1999
Kelner MJ, Bagnell R, Hale B, Alexander NM: Potential of methylene blue to block oxygen radical generation in reperfusion injury. Basic Life Sci 49: 895-898, 1988
Keaney JF, Puyana JC, Francis S, Loscalzo JF, Stamler JS, Loscalzo J: Methylene blue reverses endotoxin-induced hypotension. Circulation Res 74: 1121-1125, 1994
Han D, Sen CK, Roy S, Kobayashi MS, Tritschler HJ, Packer L: Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am J Physiol 273: R1771-R1778, 1997
Packer L, Witt EH, Tritschler HJ: Alpha-lipoic acid as a biological antioxidant. Free Rad Biol Med 19: 227-250, 1995
Müller U, Krieglstein J: Prolonged treatment with α-lipoic acid protects cultured neurons against hypoxic, glutamate-, or iron-induced injury. J Cereb Blood Flow Metabol 15: 624-630, 1995
Panigrahi M, Sadguna Y, Shivakumar BR, Kolluri SVR, Roy S, Packer L, Ravindranath V: α-lipoic acid protects against reperfusion injury following cerebral ischemia in rats. Brain Res 717: 184-188, 1996
Shi X, Ding M, Dong Z, Chen F, Ye J, Wang S, Leonard SS, Castranova V, Vallyathan V: Antioxidant properties of aspirin: Characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-κB activation, and TNF-α production. Mol Cell Biochem 199: 93-102, 1999
Wolff SP: Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Meth Enzymol 233: 182-189, 1994
Góth L: A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196: 143-152, 1991
Sturk A, van Deventer SJH, Wortel CH, Levels JHM, Ten Cate JW, Büller HR, Sanders GTB: Detection and clinical relevance of human endotoxemia. Z Med Lab Diagn 31: 147-158, 1990
Haj-Yehia AI, Assaf P, Nassar T, Katzhendler J: Determination of lipoic acid and dihydrolipoic acid in human plasma and urine by high-performance liquid chromatography with fluorimetric detection. J Chromatogr A 870: 381-388, 2000
Cordis GA, Das DK: High-performance liquid chromatographic detection of myocardial prostaglandins and thromboxanes. J Chromatogr A 536: 309-317, 1991
Colton CA, Gilbert DL: Microglia, an in vivo source of reactive oxygen species in the brain. Adv Neurol 59: 321-326, 1993
Klegeris A, McGeer PL: Rat brain microglia and peritoneal macrophages show similar responses to respiratory burst stimulants. J Neuroimmunol 53: 83-90, 1994
Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL: Measurement and characterization of superoxide generation in microglial cells: Evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys 353: 312-321, 1998
Bal-Price A, Matthias A, Brown GC: Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem 80: 73-80, 2002
Cordis GA, Das DK, Riedel W: High-performance liquid chromatographic peak identification of 2,4-dinitrophenylhydrazine derivatives of lipid peroxidation aldehydes by photodiode array detection. J Chromatogr A 798: 117-123, 1998
Fridovich I: Superoxide anion radical (O2 −), superoxide dismutases, and related matters. J Biol Chem 272: 18515-18517, 1997
McCord JM, Fridovich I: The utility of superoxide dismutase in studying free radical reactions. J Biol Chem 245: 1374-1377, 1970
Salaris SC, Babbs CF, Voorhees WD III: Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem Pharmacol 42: 499-506, 1991
Shi X, Ding M, Dong Z, Chen F, Ye J, Wang S, Leonard SS, Castranova V, Vallyathan V: Antioxidant properties of aspirin: Characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-κB activation, and TNF-α production. Mol Cell Biochem 199: 93-102, 1999
Biewenga GP, Haenen GRR, Bast A: The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29: 315-331, 1997
Chatterjee U, Sanwal GG: Purification and characterization of catalase from goat (Capra capra) lung. Mol Cell Biochem 126: 125-133, 1993
Davies KJA: Protein damage and degradation by oxygen radicals. J Biol Chem 262: 9895-9901, 1987
Maulik N, Watanabe M, Engelman D, Engelman RM, Kagan VE, Kisin E, Tyurin V, Cordis GA, Das DK: Myocardial adaptation to ischemia by oxidative stress induced by endotoxin. Am J Physiol 269: C907-C916, 1995
Riedel W: Temperature homeostasis and redox homeostasis. In: M. Kosaka, T. Suguhara, K.L. Schmidt, E. Simon (eds). Thermotherapy: Principles and Practice. Springer, Tokyo, 2001, pp 300-312
Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol 231: 232-235, 1971
Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P: Nitric oxid activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90: 7240-7244, 1993
Weihrauch D, Riedel W: Nitric oxide (NO) and oxygen radicals, but not prostaglandins, modulate fever. Ann NY Acad Sci 813: 373-382, 1997
Zenker W, Kubik S: Brain cooling in humans — anatomical considerations. Anat Embryol 193: 1-13, 1996
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA: Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47-63, 1985
Sugaya K, Chou S, Xu SJ, McKinney M: Indicators of glial activation and brain oxidative stress after intraventricular infusion of endotoxin. Mol Brain Res 58: 1-9, 1998
McNaught KSP, Jenner P: Extracellular accumulation of nitric oxide, hydrogen peroxide, and glutamate in astrocytic cultures following glutathione depletion, complex I inhibition, and/or lipopolysaccharide-induced activation. Biochem Pharmacol 60: 979-988, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riedel, W., Lang, U., Oetjen, U. et al. Inhibition of oxygen radical formation by methylene blue, aspirin, or α-lipoic acid, prevents bacterial-lipopolysaccharide-induced fever. Mol Cell Biochem 247, 83–94 (2003). https://doi.org/10.1023/A:1024142400835
Issue Date:
DOI: https://doi.org/10.1023/A:1024142400835