Abstract
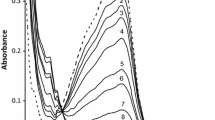
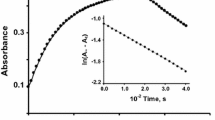
The common metal chelation agents, DTPA and EDTA are often used as models for physiological low-molecular weight iron complexes in biochemical studies, or for common biochemical protocols. In the biochemical literature there are apparent conflicts as to whether EDTA and DTPA are pro-oxidant or antioxidant additives. This apparent conflict is puzzling since in chemical systems FeIIEDTA and FeIIDTPA are well known Fenton reaction reagents. In this investigation we examined the voltammetric characteristics of the iron complexes of EDTA, DTPA, and citrate and the effect of the ligand:metal ratio (L:M) on the electrocatalytic (EC') waves that result from reduction of H2O2 by this complex. At a ratio of 1:1, the cyclic voltammetric waves of the complexes indicate the presence of a reversible species corresponding to the FeII/IIIL couple, along with a second irreversible reduction peak. The second irreversible voltammetric peak decreases at higher L:M ratios for EDTA and citrate. The 1:1 iron complexes of EDTA, DTPA, and citrate clearly induce the catalytic reduction of H2O2. In the presence of a greater than 100 fold excess of H2O2 relative to iron, higher L:M ratios greatly reduced the catalytic EC' wave compared to the 1:1 ratios. At H2O2:Fe ratios less than 50, the L:M ratio has very little effect of the EC' current. These observations may explain the apparent discrepancies in the biochemical literature. Addition of EDTA or DTPA may enhance oxidative processes if the L:M is low (less than unity), whereas rates of on-going oxidative processes may decrease if that ratio, along with the relative amount of H2O2, are both high (excess ligand). The impact of this study is of particular importance given the widespread use of these ligands in biochemical studies.
Similar content being viewed by others
References
Alayash AI, Patel RP, Cashon RE. 2001 Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid. Redox Signal 3(2), 313–327.
Ansari NH, Wang L, Erwin AA, Church DF. 1996 Glucosedependent formation of free radical species in lens homogenate. Biochem Mol Med 59, 68–71.
Aoki K, Ishida M, Tokuda K. 1988 Voltammetry at microcylinder electrodes Part IV: Second-order catalytic reaction of ironethylenediamminetetraacetic acid with hydrogen peroxide. J Electroanal Chem 245, 39–50.
Babior BM. 2000 Phagocytes and oxidative stress. Am J Med 109, 33–44.
Bard AJ, Faulkner LR. 2001 In: Electrochemical Methods: Fundamentals and Applications, 2nd Edition. New York: John Wiley and Sons; 501.
Berlett BS, Chock PB, Yim MB, Stadtman ER. 1990 Manganese(II)-bicarbonate-mediated catalytic activity for hydrogen peroxide dismutation and amino acid oxidation: Detection of free radical intermediates. Proc Nat Acad Sci USA 87(1), 389–393.
Berman SB, Zigmond MJ, Hastings TG. 1996 Modification of dopamine transporter function: effect of reactive oxygen species and dopamine. J Neurochem 67, 593–600.
Berry MJ, Brosnan J, Fennell CA, Hamilton AF, Dominiczak C. 2001 Oxidative stress and vascular damage in hypertension. Curr Opin Nephrol Hypertens 10, 247–255.
Buettner GR, Jurkiewicz BA. 1996 Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res 145, 532–541.
Burkitt MJ, Gilbert BC. 1990 Model studies of the iron-catalysed Haber-Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic Res Commun 10, 265–280.
Cantin-Esnault D, Oubrahim H, Richard JM. 2000 DNA strand scission by the nephrotoxin [2,2′-bipyridine]-3,3′,4,4′-tetrol-1,1′-dioxide and related compounds in the presence of iron. Free Radic Res 33, 129–137.
Chan PH. 2001 Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21, 2–14.
Cox J, Cummings TE. 1973 Cyclic voltammery of the iron (III)/(II) couple in citrate and phosphate media. J Electroanal Chem 42, 153–157.
Crichton RR. 1987 Iron metabolism and oxygen toxicity. Bioelectrochem Bioenerg 18, 105–116.
Crichton RR, Ward RJ. 1992 Iron metabolism – new perspectives in view. Biochemistry 31, 11255–11264.
Dabbagh AJ, Trenam CW, Morris CJ, Blake DR. 1993 Iron in joint inflammation. Ann Rheum Diseases 52, 67–73.
Egan TJ, Barthakur SR, Aisen P. 1992 Catalysis of the Haber-Weiss reaction by irondiethylenetriaminepentaacetate. J Inorg Biochem 48, 241–249.
Escot MT, Martre AM, Pouillen P, Martinet P. 1989 Electrochemical stduy of iron (III) complexation by some model ligands of biological interest. I. Acetohydroxamic acid, acetylacetone, and citric acid. Bull Soc Chim Fr 3, 316–320.
Evans RK, Xu Z, Bohannon KE, Wang B, Bruner MW, Volkin DB. 2000 Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J Pharm Sci 89, 76–87.
Fojta M, Kubicarova T, Palecek E. 2000 Electrode potentialmodulated cleavage of surface-confined DNA by hydroxyl radicals detected by an electrochemical biosensor. Biosens Bioelectron 15, 107–115.
Galley HF, Webster NR. 1996 Elevated serum bleomycin-detectable iron concentrations in patients with sepsis syndrome. Intensive Care Med 22, 226–229.
Gans P, Sabatini A, Vacca A. 2000 Hyperquad Simulation and Speciation Equilibrium Modeling Software.
Gordan LI, Wietzman SA. 1988 The Respiratory Burst and its Physiological Significance. In: Sbarra AJ, Strauss RR, eds. The Respiratory Burst and Carcinogenesis. New York: Plenum Press; 277–298.
Gutteridge JM. 1986 Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett 201, 291–295.
Gutteridge JM. 1994 Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact 91, 133–140
Gutteridge JMC, Mumby GJ, Quinlan GJ, Chung KF, Evans TW. 1996 Pro-oxidant iron is present in human pulmonary epithelial lining fluid: implications for oxidative stress in the lung. Biochem Biophys Res Comm 220, 1024–1027.
Gutteridge JMC, Mumby S, Koizumi M, Taniguchi N. 1996 'Free’ iron in neonatal plasma activates aconitase: evidence for biologically reactive iron. Biochem Biophys Res Comm 229, 806–809.
Halliwell B, Arouma OI. 1991 DNA damage by oxygen-derived species. Its mechanism and measurement inmammalian systems. FEBS Lett 281, 9–19.
Halliwell B, Gutteridge JMC. 1989 Free Radicals in Biology and Medicine, New York: Oxford University Press; 36.
Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. 2000 Redox regulatory mechanisms of cellular signal transduction. Free Radic Biol Med 28, 1456–1462.
Jacobs A. 1977 Serum ferritin and iron stores. Blood 50, 433–439.
Jung M, Drapier JC, Weidenbach H et al. 2000 Effects of hepatocellular iron imbalance on nitric oxide and reactive oxygen intermediates production in a model of sepsis. Hepatology 33, 387–394.
Kachur AV, Tuttle SW, Bigalow JE. 1998 Autoxidation of ferrous ion complexes: a method for the generation of hydroxyl radicals. Radiat Res 150, 475–482.
Kaim W, Schwederski B. 1996 Bioinorganic chemistry: Inorganic elements in the chemistry of life. New York: John Wiley and Sons; 8.
Kaneko H, Nozaki K, Ozawa T. 1978 Electrochemical estimation of reactivities of OH Radical produced in Fe-EDTA-H2O2 system. J Electroanal Chem 87, 149–153.
Kehrer JP. 2000 The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 49, 43–50.
Koppenol WH. 1987 The Haber-Weiss cycle – 71 years later. Bioelectrochem Bioenerg 18, 3–11.
Kovacic PD Jacintho J. 2001 Mechanisms of carcinogenesis: Focus on oxidative stress and electron transfer. Curr Med Chem 8, 863–892.
Kowald A. 2001 The mitochondrial theory of aging. Biol Signals Recept 10, 162–175.
Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. 2000 Role of redoxregulated transcription factors in inflammation, aging and agerelated diseases. Exp Gerontol 35, 521–532.
Lee SH, Yoon YC, Jang YY, Song JH, Han ES, Lee CS. 2001 Effect of iron and ascorbate on cyclosporine-induced oxidative damage of kidney mitochondria and microsomes. Pharmacol Res 43, 161–171.
Lum H, Roebuck KA. 2001 Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280, C719–C741.
Neese F, Solomon EI. 1998 Detailed spectroscopic and theoretical studies on [Fe(EDTA)(O2)]3-: Electronic structure of the side-on ferric-peroxide bond and it's relevance to reactivity. J Am Chem Soc 120, 12829–12848.
Novellino L, Napolitano A, Prota G. 1999 5,6-Dihydroxyindoles in the fenton reaction: a model study of the role of melanin precursors in oxidative stress and hyperpigmentary processes. Chem Res Toxicol 12, 985–992.
Patruta SI, Horl WH. 1999 Iron and infection. Kidney Int Suppl 69, S125–S130.
Penta JS, Johnson FM, Wachsman JT, Copeland WC. 2001 Mitochondrial DNA in human malignancy. Mutat Res 488, 119–133.
Peskin AV. 1996 Nuclear DNA damage during NAD(P)H oxidation by membrane redox chains. Free Radic Biol Med 20, 313–318.
Pierre JL, Fontacave M. 1999 Iron and activated oxygen species in biology: The basic chemistry. Biometals 12, 195–199.
Pierre JL, Gautier-Luneau I. 2000 Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance. BioMetals 13, 91–96.
Ramakrishna Rao DN, Cederbaum AI. 1996 Generation of reactive oxygen species by the redox cycling of nitroprusside. Biochim Biophys Acta 1289, 195–202.
Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. 2000 In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease: a central role for bound transition metals. J Neurochem 74, 270–279.
Smith RM, Martell AE. 1975 Critical Stability Constants, Vols. 1–4. New York: Plenum Press.
Sohn SC, Suh MY, Eom TY. 1993 Polarographic determinations of iron(II), iron(III) and total iron in the presence of DTPA. J Korean Chem Soc 37, 1053–1059.
Stadtman ER, Berlett BS. 1991 Fenton chemistry. Amino acid oxidation. J Biologic Chem 266, 17201–17211.
Stolze K, Udilova N, Nohl H. 2000 ESR analysis of spin adducts of alkoxyl and lipid-derived radicals with the spin trap Trazon. Free Radic Biol Med 29, 1005–1014.
Stulikova M, Vydra F. 1972 Voltammetry with disk electrodes and its application: Voltammetry of iron (III) at the glassy carbon rotating disk electrode in complexing media. J Electroanal Chem 39, 229–231.
Verma PS, Saxena RC, Jayaraman A. 1997 Cyclic voltammetric studies of certain industrially potential iron chelate catalysis. Frensenius J Anal Chem 357, 56–60.
Walling C, Kurz M, Schugar HJ. 1970 The iron(III)-ethylenediaminetetraacetic acid-peroxide system. Inorg Chem 9/4, 931–937.
Wattanapitayakul SK, Bauer JA. 2001 Oxidative pathways in cardiovascular disease: Roles, mechanisms, and therapeutic implications. Pharmacol Ther 89, 187–206.
Yamazaki I, Piette LH. 1990 ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem 265, 13589–13594.
Yoo YM, Kim KM, Kim SS, Han JA, Lea HZ, Kim YM. 1999 Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling. Clin Diagn Lab Immunol 6, 938–945.
Yoon SJ, Koh YH, Floyd RA, Park JW. 2000 Copper, zinc superoxide dismutase enhances DNA damage and mutagenicity induced by cysteine/iron. Mutat Res 448, 97–104.
Zhao F, Yang J, Schoneich C. 1996 Effects of polyaminocarboxylate metal chelators on iron-thiolate induced oxidation of methionineand histidine-containing peptides. Pharm Res 13, 931–918.
Zhuang Q, Chen H. 1993 A theoretical analysis and its application of the second order EC’ reactions at microelectrodes under steady-state conditions. Chin J Chem 11, 308–315.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Engelmann, M.D., Bobier, R.T., Hiatt, T. et al. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. Biometals 16, 519–527 (2003). https://doi.org/10.1023/A:1023480617038
Issue Date:
DOI: https://doi.org/10.1023/A:1023480617038