Abstract
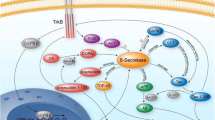
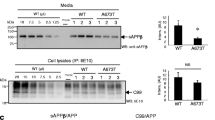
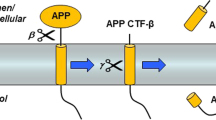
Secretases degrade amyloid precursor protein (APP) releasing fragments (β-peptides Aβ, Aβx) that assemble to form hallmark extracellular deposits in Alzheimer's disease (AD) correlating with disease severity. As such, secretases supply targets for therapeutic intervention and form the focus of this overview. Progress in elucidating secretases and their modes of catalysis come from exploiting the use of transgenics or transfected cells. In addition to Aβx, secretases also release C-terminal fragments with putative signaling properties (amyloid intracellular domain, AICD) similar in concept to those available for conversion of the Notch-r to release the nuclear transactivator NICD. The review considers lingering questions on APP fragmentation by secretase action, ancillary proteins such as presenilins (PS1/2), nicastrin, XII, or proteases (caspases), and the influence of familial mutations (mAPP, mPS) in terms of fibrillogenesis.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marks, N., Berg, M.J. APP Processing Enzymes (Secretases) as Therapeutic Targets: Insights from the Use of Transgenics (Tgs) and Transfected Cells. Neurochem Res 28, 1049–1062 (2003). https://doi.org/10.1023/A:1023211323853
Issue Date:
DOI: https://doi.org/10.1023/A:1023211323853