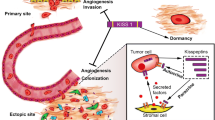
Abstract
Metastatic disease is the most critical impediment to cancer patient survival. However, comparatively little is known concerning the intricate pathways which govern the complex phenotypes associated with metastasis. The KISS1 metastasis suppressor gene inhibits metastasis in both in vivo melanoma and breast carcinoma models. Despite its clear physiological activity, the mechanism of KISS1 remains unclear. Recent identification of a 54 amino acid peptide of KISS1, termed metastin or kisspeptin-54, and its cognate G-protein coupled receptor (hOT7T175, AXOR12, GPR54) have provided additional clues and avenues of research. While studies have attributed KISS1 with modulation of NFκB regulation, experiments with metastin and its receptor implicate MAP kinase pathways and also suggest the potential of autocrine, paracrine and endocrine roles. Impacts on motility, chemotaxis, adhesion and invasion have each been documented in disparate cell lines and conflicting observations require resolution. Nevertheless, mounting clinical evidence, particularly the loss of KISS1 in metastases, correlates KISS1 and metastin receptor expression with human tumor progression. Together, the data substantiate roles for these molecules in metastasis regulation.
Similar content being viewed by others
References
Bailly M, Zebda N, Dore JF. Human melanoma metastasis related to specific adhesion with lung cells rather than direct growth stimulation. Anticancer Res 1994; 14(5A): 1791–9.
Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L (eds): Melanomas of skin. SEER Cancer Statistics Review, 1973-1998. Bethesda: National Cancer Institute, 2001.
Welch DR, Bisi JE, Miller BE et al. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer 1991; 47(2): 227–37.
Miele ME, Robertson G, Lee JH et al. Metastasis suppressed, but tumorigenicity and local invasiveness unaffected, in the human melanoma cell line MelJuSo after introduction of human chromosomes 1 or 6. Mol Carcinog 1996; 15(4): 284–99.
Miele ME, Jewett MD, Goldberg SF et al. A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer 2000; 86(4): 524–8.
Welch DR, Chen P, Miele ME et al. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene 1994; 9(1): 255–62.
Lee JH, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer 1997; 71(6): 1035–44.
Lee JH, Miele ME, Hicks DJ et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996; 88(23): 1731–7.
Shirasaki F, Takata M, Hatta N et al. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res 2001; 61(20): 7422–5.
Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res 1997; 57(12): 2384–7.
West A, Vojta PJ, Welch DR et al. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics 1998; 54(1): 145–8.
Gualandi F, Morelli C, Pavan JV et al. Induction of senescence and control of tumorigenicity in BK virus transformed mouse cells by human chromosome 6. Genes Chromosomes Cancer 1994; 10(2): 77–84.
Morelli C, Sherratt T, Trabanelli C et al. Characterization of a 4-Mb region at chromosome 6q21 harboring a replicative senescence gene. Cancer Res 1997; 57(19): 4153–7.
Goldberg SF, Miele ME, Welch DR. VDUP1 mediates human melanoma metastasis suppression by chromosome 6. Proc Am Assoc Cancer Res 2002; 43: 138.
Junn E, Han SH, Im JY et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 2000; 164(12): 6287–95.
PROSITE. http://www.expasy.ch: 2001.
SignalP v1.1. http://www.cbs.dtu.dk/services/SignalP/: 2001.
PSORT. http://psort.nibb.ac.jp: 2001.
PESTfind. http://www.at.embnet.org/embnet/tools/bio/PESTfind: 2001.
Ohtaki T, Shintani Y, Honda S et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411(6837): 613–7.
Kotani M, Detheux M, Vandenbogaerde A et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276(37): 34631–6.
Muir AI, Chamberlain L, Elshourbagy NA et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001; 276(31): 28969–75.
Shi CS, Kehrl JH. PYK2 links G(q)alpha and G(13)alpha signaling to NF-kappa B activation. J Biol Chem 2001; 276(34): 31845–50.
Ringel MD, Hardy E, Bernet VJ et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab 2002; 87(5): 2399.
Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem 2001; 276(2): 1164–72.
You J, Miele ME, Dong C et al. Suppression of human melanoma metastasis by introduction of chromosome 6 may be partially due to inhibition of motility, but not to inhibition of invasion. Biochem Biophys Res Commun 1995; 208(2): 476–84.
Strasberg RM, Welch DR, Rieber M. Suppression of C8161 melanoma metastatic ability by chromosome 6 induces differentiation-associated tyrosinase and decreases proliferation on adhesion-restrictive substrates mediated by overexpression of p21WAF1 and down-regulation of bcl-2 and cyclin D3. Biochem Biophys Res Commun 2001; 281(1): 159–65.
Hori A, Honda S, Asada M et al. Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun 2001; 286(5): 958–63.
Goldberg SF, Harms JF, Quon K et al. Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin Exp Metastasis 1999; 17(7): 601–7.
Harms JF, Miele ME, Welch DR. Metastasis suppression of C8161 human melanoma cells by chromosome 6 appears to be organ specific. Proc Am Assoc Cancer Res 2002; 43: 138–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harms, J.F., Welch, D.R. & Miele, M.E. KISS1 metastasis suppression and emergent pathways. Clin Exp Metastasis 20, 11–18 (2003). https://doi.org/10.1023/A:1022530100931
Issue Date:
DOI: https://doi.org/10.1023/A:1022530100931