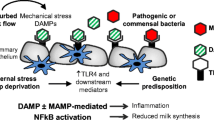
Abstract
Lactation is considered the final phase of the mammalian reproductive cycle, and the mammary gland provides milk for nourishment and disease resistance to the newborn. However, the cellular and soluble immune components associated with mammary tissues and secretion also can play an important role in protecting the gland from infectious diseases, such as mastitis. Mastitis can affect essentially all lactating mammals, but is especially problematic for dairy cattle. The most recent estimates from the National Mastitis Council suggest that mastitis affects one third of all dairy cows and will cost the dairy industry over 2 billion dollars annually in the United States in lost profits (National Mastitis Council (1996) Current Concepts in Bovine Mastitis, National Mastitis Council, Madison, WI). The overall impact of mastitis on the quality and quantity of milk produced for human consumption has provided the impetus to better understand the pathophysiology of the mammary gland and develop ways to enhance disease resistance through immunoregulation. As such, the bovine species has played a critical and prominent role in our current understanding of mammary gland immunobiology. This paper provides a comprehensive overview of mammary gland immunity and how the stage of lactation can impact important host defenses. While this review emphasizes the bovine system, comparisons to humans and other domestic mammals will be addressed as well.
Similar content being viewed by others
REFERENCES
R. I. Lehrer and T. Ganz (1999). Antimicrobial peptides in mammalian and insect host defence. Curr.Opin.Immunol. 11: 23–27.
E. Telemo and L.A. Hanson (1996). Antibodies in milk. J.Mam. Gland Biol.Neoplasia 1: 243–249.
D. Filipp, K. Alizadeh-Khiavi, C. Richardson, A. Palma, N. Paredes, O. Takeuchi, S. Akira, and M. Julius (2001). Sol-uble CD14 enriched in colostrum and milk induces B cell growth and differentiation. Proc.Natl.Acad.Sci.USA 98: 603–608.
L. M. Sordillo, K. Shafer-Weaver, and D. de Rosa (1997). Im-munobiology of the mammarygland. J.Dairy Sci. 80: 1851–1865.
L. A. Hanson (2000). The mother–offspring dyad and the im-mune system. Acta Paediatr. 89: 252–258.
L. Zhou, Y. Yoshimura, Y. Y. Huang, R. Suzuki, M. Yokoyama, M. Okabe, and M. Shimamura (2000). Two independent path-ways of maternal cell transmission to offspring: Through pla-centa during pregnancy and by breast-feeding after birth. Immunology 101: 570–580.
J. H. Nuijens, P. H. van Berkel, and F. L. Schanbacher (1996). Structure and biological actions of lactoferrin. J.Mam.Gland Biol.Neoplasia 1: 285–295.
B. L. Larson (1992). Immunoglobulins of the mammary secre-tions. In P. F. Fox (ed.), Advanced Dairy Chemistry, Vol: 1 Pro-teins, Elsevier, London, pp. 231–254.
W. Hunziker and J. P. Kraehenbuhl (1998). Epithelial transcyto-sis of immunoglobulins. J.Mam.Gland Biol.Neoplasia 3: 287–302.
E. C. Butcher and L. J. Picker (1996). Lymphocyte homing and homeostasis. Science 272: 60–66.
F. E. Johansen, R. Braathen, and P. Brandtzaeg (2000). Role of J chain in secretory immunoglobulin formation. Scand.J. Immunol. 52: 240–248.
F. E. Johansen, M. Pekna, I. N. Norderhaug, B. Haneberg, M. A. Hietala, P. Krajci, C. Betsholtz, and P. Brandtzaeg (1999). Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglob-ulin receptor/secretory component-deficient mice. J.Exp.Med. 190: 915–922.
G. M. Barrington, T. B. McFaden, M. T. Huyler, and T. E. Besser (2001). Regulation of colostrogenesis in cattle. Livestock Prod. Sci. 70: 95–104.
E. J. Israel, V. K. Patel, S. F. Taylor, A. Marshak-Rothstein, and N. E. Simister (1995). Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J.Immunol. 154: 6246–6251.
D. Velin, H. Acha-Orbea, and J. P. Kraehenbuhl (1996). The neonatal Fc receptor is not required for mucosal infection by mouse mammary tumor virus. J.Virol. 70: 7250–7254.
G. M. Barrington, T. E. Besser, W. C. Davis, C. C. Gay, J. J. Reeves, and T. B. McFadden (1997). Expression of im-munoglobulin G1receptors by bovine mammaryepithelial cells and mammary leukocytes. J.Dairy Sci. 80: 86–93.
G. M. Barrington, T. E. Besser, C. C. Gay, W. C. Davis, J. J. Reeves, and T. B. McFadden (1997). Effect of prolactin on in vitro expression of the bovine mammary immunoglobulin G1 receptor. J.Dairy Sci. 80: 94–100.
C. L. Lamprecht, H. E. Krause, and M. A. Mufson (1976). Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J.Infect.Dis. 134: 211–217.
L. Enjuanes and B. A. M. van der Zeijst (1995). Molecular ba-sis of transmissible gastroenteritis virus epidemiology. In S. G. Siddell (ed.), The Coronaviridae, Plenum Press, New York, pp. 337–376.
M. C. Jenkins, C. O'Brien, J. Trout, A. Guidry, and R. Fayer (1999). Hyperimmune bovine colostrum specific for recombi-nant Cryptosporidium parvum antigen confers partial protec-tion against cryptosporidiosis in immunosuppressed adult mice. Vaccine 17: 2453–2460.
W. Stephan, H. Dichtelmuller, and R. Lissner (1990). Antibod-ies from colostrum in oral immunotherapy. J.Clin.Chem.Clin. Biochem. 28: 19–23.
H. Korhonen, P. Marnila, and H. S. Gill (2000). Milk im-munoglobulins and complement factors. Br.J.Nutr. S75–S80.
H. Korhonen, P. Marnila, and H. S. Gill (2000). Bovine milk antibodies for health. Br.J.Nutr. S135–S146.
V. Loimaranta, J. Nuutila, P. Marnila, J. Tenovuo, H. Korhonen, and E. M. Lilius (1999). Colostral proteins from cows immu-nised with Streptococcus mutans/S. sobrinus support the phago-cytosis and killing of mutans streptococci by human leucocytes. J.Med.Microbiol. 48: 917–926.
Y. Okamoto, H. Tsutsumi, N. S. Kumar, and P. L. Ogra (1989). Effect of breast feeding on the development of anti-idiotype antibody response to F glycoprotein of respiratory syncytial virus in infant mice after post-partum maternal immunization. J.Immunol. 142: 2507–2512.
D. P. Pollock, J. P. Kutzko, E. Birck-Wilson, J. L. Williams, Y. Echelard, and H. M. Meade (1999). Transgenic milk as a method for the production of recombinant antibodies. J.Immunol.Methods 231: 147–157.
I. Sola, J. Castilla, B. Pintado, J. M. Sanchez-Morgado, C. B. Whitelaw, A. J. Clark, and L. Enjuanes. (1998). Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J.Virol. 72: 3762–3772.
P. Zhang, V. Sawicki, A. Lewis, L. Hanson, J. H. Nuijens, and M. C. Neville (2001). Human lactoferrin in the milk of trans-genic mice increases intestinal growth in ten-day-old suckling neonates. Adv.Exp.Med.Biol. 501: 107–113.
G. J. Platenburg, E. P. Kootwijk, P. M. Kooiman, S. L. Woloshuk, J. H. Nuijens, P. J. Krimpenfort, F. R. Pieper, H. A. de Boer, and R. Strijker (1994). Expression of human lactoferrin in milk of transgenic mice. Transgenic Res. 3: 99–108.
S. J. Kim, B. H. Sohn, S. Jeong, K. W. Pak, J. S. Park, I. Y. Park, T. H. Lee, Y. H. Choi, C. S. Lee, Y. M. Han, D. Y. Yu, and K. K. Lee (1999). High-level expression of human lactoferrin in milk of transgenic mice using genomic lactoferrin sequence. J.Biochem.(Tokyo) 126: 320–325.
S. Yarus, J. M. Rosen, A. M. Cole, and G. Diamond (1996). Production of active bovine tracheal antimicrobial peptide in milk of transgenic mice. Proc.Natl.Acad.Sci.USA 93: 14118–14121.
E. A. Maga, G. B. Anderson, and J. D. Murray (1995). The effect of mammary gland expression of human lysozyme on the properties of milk from transgenic mice. J.Dairy Sci. 78: 2645–2652.
E. A. Maga, G. B. Anderson, J. S. Cullor, W. Smith, and J. D. Murray (1998). Antimicrobial properties of human lysozyme transgenic mouse milk. J.Food Prot. 61: 52–56.
D. E. Kerr, K. Plaut, A. J. Bramley, C. M. Williamson, A. J. Lax, K. Moore, K. D. Wells, and R. J. Wall (2001). Lysostaphin ex-pression in mammary glands confers protection against staphy-lococcal infection in transgenic mice. Nat.Biotechnol. 19: 66–70.
A. F. Kolb, L. Pewe, J. Webster, S. Perlman, C. B. Whitelaw, and S. G. Siddell (2001). Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis. J.Virol. 75: 2803–2809.
A. J. Clark, A. Cowper, R. Wallace, G. Wright, and J. P. Simons (1992). Rescuing transgene expression by co-integration. Biotechnology 10: 1450–1454.
K. W. Dobie, M. Lee, J. A. Fantes, E. Graham, A. J. Clark, A. Springbett, R. Lathe, and M. McClenaghan (1996). Variegated transgene expression in mouse mammary gland is determined by the transgene integration locus. Proc.Natl.Acad.Sci.USA 93: 6659–6664.
A. F. Kolb, R. Ansell, J. McWhir, and S. G. Siddell (1999). In-sertion of a foreign gene into the beta-casein locus by Cre-mediated site-specific recombination. Gene 227: 21–31.
N. de Groot, P. van Kuik-Romeijn, S. H. Lee, and H. A. de Boer (2000). Increased immunoglobulin A levels in milk by over-expressing the murine polymeric immunoglobulin recep-tor gene in the mammary gland epithelial cells of transgenic mice. Immunology 101: 218–224.
P. A. Furth (1997). Conditional control of gene expression in the mammary gland. J.Mam.Gland Biol.Neoplasia 2: 373–383.
S. Soulier, M. G. Stinnakre, L. Lepourry, J. C. Mercier, and J. L. Vilotte (1999). Use of doxycycline-controlled gene expression to reversibly alter milk-protein composition in transgenic mice. Eur.J.Biochem. 260: 533–539.
A. Jakobovits (1995). Production of fully human antibodies by transgenic mice. Curr.Opin.Biotechnol. 6: 561–566.
K. Tomizuka, T. Shinohara, H. Yoshida, H. Uejima, A. Ohguma, S. Tanaka, K. Sato, M. Oshimura, and I. Ishida (2000). Double trans-chromosomic mice: Maintenance of two individ-ual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc.Natl. Acad.Sci.USA 97: 722–727.
A. Jakobovits (1998). Production and selection of antigen-specific fully human monoclonal antibodies from mice engi-neered with human Ig loci. Adv.Drug Deliv.Rev. 31: 33–42.
D. S. Newburg (1996). Oligosaccharides and glycoconjugates in human milk: Their role in host defense. J.Mam.Gland Biol. Neoplasia 1: 271–283.
A. S. Goldman, S. Chheda, R. Garofalo, and F. C. Schmalstieg (1996). Cytokines in human milk: Properties and potential ef-fects upon the mammary gland and the neonate. J.Mam.Gland Biol.Neoplasia 1: 251–258.
P. M. Hwang, N. Zhou, X. Shan, C. H. Arrowsmith, and H. J. Vogel (1998). Three-dimensional solution structure of lacto-ferricin B, an antimicrobial peptide derived from bovine lacto-ferrin. Biochemistry 37: 4288–4298.
S. Baveye, E. Elass, J. Mazurier, G. Spik, and D. Legrand (1999). Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin.Chem.Lab.Med. 37: 281–286.
B. W. van der Strate, L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer (2001). Antiviral activities of lactoferrin. An-tiviral Res. 52: 225–239.
P. Valenti, R. Greco, G. Pitari, P. Rossi, M. Ajello, G. Melino, and G. Antonini (1999). Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: Protective effect of lacto-ferrin. Exp.Cell Res. 250: 197–202.
T. Wada, Y. Aiba, K. Shimizu, A. Takagi, T. Miwa, and Y. Koga (1999). The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand.J.Gastroenterol. 34: 238–243.
L. A. Hanson (1998). Breastfeeding provides passive and likely long-lasting active immunity. Ann.Allergy Asthma Immunol. 81: 523–533.
A. F. Kolb (2001). The prospects of modifying the antimicrobial properties of milk. Biotechn.Adv. 19: 299–316.
L. Edde, R. B. Hipolito, F. F. Hwang, D. R. Headon, R. A. Shalwitz, and M. P. Sherman (2001). Lactoferrin protects neonatal rats from gut-related systemic infection. Am.J.Phys-iol. Gastrointest.Liver Physiol. 281: G1140–G1150.
L. H. Hanson, V. Sawicki, A. Lewis, J. H. Nuijens, M. C. Neville, and P. Zhang (2001). Does human lactoferrin in the milk of transgenic mice deliver iron to suckling neonates? Adv.Exp. Med.Biol. 501: 233–239.
P. Krimpenfort, A. Rademakers, W. Eyestone, A. van der Schans, S. van den Broek, P. Kooiman, E. Kootwijk, G. Platenburg, F. Pieper, R. Strijker, and H. A. de Boer (1991). Generation of transgenic dairy cattle using ‘in vitro’ embryo production. Biotechnology (NY) 9: 844–847.
P. M. Hwang and H. J. Vogel (1998). Structure–function rela-tionships of antimicrobial peptides. Biochem.Cell Biol. 76: 235–246.
W. Bellamy, M. Takase, H. Wakabayashi, K. Kawase, and M. Tomita (1992). Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J.Appl.Bacteriol. 73: 472–479.
H. Ulvatne, H. H. Haukland, O. Olsvik, and L. H. Vorland (2001). Lactoferricin B causes depolarization of the cytoplas-mic membrane of Escherichia coli ATCC 25922 and fusion of negatively charged liposomes. FEBS Lett. 492: 62–65.
R. Drews, R. K. Paleyanda, T. K. Lee, R. R. Chang, A. Rehemtulla, R. J. Kaufman, W. N. Drohan, and H. Lubon (1995). Proteolytic maturation of protein C upon engineering the mouse mammary gland to express furin. Proc.Natl.Acad. Sci.USA 92: 10462–10466.
H. D. Zucht, M. Raida, K. Adermann, H. J. Magert, and W. G. Forssmann (1995). Casocidin-I: Acasein-alpha s2 derived peptide exhibits antibacterial activity. FEBS Lett. 372: 185–188.
I. Recio and S. Visser (1999). Identification of two distinct an-tibacterial domains within the sequence of bovine alpha(s2)-casein. Biochim.Biophys.Acta 1428: 314–326.
A. Pellegrini, U. Thomas, N. Bramaz, P. Hunziker, and R. von Fellenberg (1999). Isolation and identification of three bac-tericidal domains in the bovine alpha-lactalbumin molecule. Biochim.Biophys.Acta 1426: 439–448.
E. Lahov and W. Regelson (1996). Antibacterial and immuno-stimulating casein-derived substances from milk: Casecidin, is-racidin peptides. Food Chem.Toxicol. 34: 131–145.
H. P. Jia, T. Starner, M. Ackermann, P. Kirby, B. F. Tack, and P. B. J. McCray (2000). Abundant human beta-defensin-1 ex-pression in milk and mammary gland epithelium. J.Pediatr. 138: 109–112.
A. Tossi, C. Tarantino, and D. Romeo (1997). Design of syn-thetic antimicrobial peptides based on sequence analogy and amphipathicity. Eur.J.Biochem. 250: 549–558.
P. Tzou, E. de Gregorio, and B. Lemaitre (2002). How Drosophila combats microbial infection: A model to study in-nate immunity and host–pathogen interactions. Curr.Opin. Microbiol. 5: 102–110.
B. C. Schutte, J. P. Mitros, J. A. Bartlett, J. D. Walters, H. P. Jia, M. J. Welsh, T. L. Casavant, and P. B. J. McCray (2002). Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc.Natl.Acad.Sci.USA 99: 2129–2133.
M. Hamosh (1998). Protective function of proteins and lipids in human milk. Biol.Neonate 74: 163–176.
K. D. Kussendrager and A. C. van Hooijdonk (2000). Lactoper-oxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br.J.Nutr. S19–S25.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sordillo, L.M., Streicher, K.L. Mammary Gland Immunity and Mastitis Susceptibility. J Mammary Gland Biol Neoplasia 7, 135–146 (2002). https://doi.org/10.1023/A:1020347818725
Issue Date:
DOI: https://doi.org/10.1023/A:1020347818725