Abstract
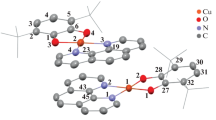
Bis(1,4-di-tert-butyl-1,4-diazabutadiene)copper(i) [(3,6-di-tert-butyl-o-benzosemiquinono)(3,6-di-tert-butylcatecholato)cuprate(ii)] (1) was synthesized. Complex 1 contains the 1,4-di-tert-butyl-1,4-diazabutadiene and 3,6-di-tert-butyl-o-benzoquinone ligands in the reduced form. The structure of 1 was established by X-ray diffraction analysis. The ESR spectra indicate that dissolution of complex 1 in organic solvents (toluene, THF, CH2Cl2, etc.) leads to its symmetrization to give neutral complex 2, which occurs in solutions as an equilibrium mixture of two redox isomers, viz., catecholate (Cat) complex 2c and semiquinone (SQ) complex 2s. In the coordination sphere of the copper atom, the reversible intramolecular metal—ligand electron transfer can proceed as successive steps as exemplified by the reactions of 2 with CO and 2,6-dimethylphenylisonitrile. Copper(i) o-semiquinone complex 2s can be reversibly transformed into copper(ii) catecholate complex 2c through electron transfer from the copper(i) atom to the SQ ligand. The subsequent addition of the neutral ligand (CO or CNAr) to 2c induces, in turn, electron transfer from the Cat ligand to the copper(ii) atom accompanied by the transformation of the catecholate complex into the o-semiquinone complex. In the case of CO, this transformation is also reversible and is efficiently controlled by the temperature.
Similar content being viewed by others
References
W. Kaim and J. Rall, Angew. Chem., Int. Ed. Engl., 1996, 35, 43.
P. Chaudhur, M. Hess, T. Weyhermuller, and K. Wieghardt, Angew. Chem., Int. Ed. Engl., 1999, 38, 1095.
P. Chaudhur, M. Hess, J. Muller, K. Hildenbrand, E. Bill, T. Weyhermuller, and K. Wieghardt, J. Am. Chem. Soc., 1999, 121, 9599.
M. Hess, T. Weyhermuller, K. Wieghardt, and P. Chaudhur, J. Inorg. Biochem., 1999, 74, 93.
G. A. Abakumov, V. K. Cherkasov, and A. V. Lobanoв, Dokl. Akad. Nauk SSSR, 1982, 266, 361 [Dokl. Chem., 1982 (Engl. Transl.)].
G. A. Abakumov, V. K. Cherkasov, V. A. Garnov, and V. I. Nevodchikov, Dokl. Akad. Nauk SSSR, 1989, 304, 107 [Dokl. Chem., 1989 (Engl. Transl.)].
J. Rall, M. Wanner, M. Albrecht, F. M. Hornung, and W. Kaim, Chem. Eur. J., 1999, 5, 2809.
Yu. N. Saf'yanov, L. N. Zakharov, Yu. T. Struchkov, V. A. Garnov, V. K. Cherkasov, and G. A. Abakumov, Koord. Khim., 1990, 16, 802 [Sov. J. Coord. Chem., 1990, 16 (Engl. Transl.)].
C. G. Pierpont and R. M. Buchanan, Coord. Chem. Rew., 1981, 38, 45.
B. A. Goodman and J. B. Raynor, Adv. Inorg. Chem. Radoichemistry, 1970, 13, 135.
R. G. Buchanan, C. Wilson-Blumenberg, and C. Trapp, Inorg. Chem., 1986, 25, 3070.
G. A. Abakumov, V. K. Cherkasov, V. I. Nevodchikov, V. A. Kuropatov, G. T. Yee, and C. G. Pierpont, Inorg. Chem., 2001, 40, 2434.
G. A. Razuvaev, G. A. Abakumov, and V. K. Cherkasov, J. Organometal. Chem., 1978, 160, 361.
G. A. Abakumov and V. K. Cherkasov, Metalloorg. Khim., 1982, 266, 361 [Organomet. Chem. USSR, 1982, 266, No. 2 (Engl. Transl.)].
Yu. V. Karyakin and I. I. Angelov, Chistye khimicheskie veshchestva [Pure Chemical Compounds], Khimiya, Moscow, 1974, 240 pp. (in Russian).
H. Dieck and I. W. Renk, Chem. Ber., 1971, 104, \(N {\underset - o}\)2, 92.
V. A. Muraev, G. A. Abakumov, and G. A. Razuvaev, Dokl. Akad. Nauk SSSR, 1974, 217, 1083 [Dokl. Chem., 1974 (Engl. Transl.)].
G. M. Sheldrick, SHELXS 86, Program for the Solution of Crystal Structures, Universitate of Göttihgen, Germany, 1985.
G. M. Sheldrick, SHELXL 93, Program for the Refinement for the Crystal Structures, Universitate of Göttihgen, Germany, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abakumov, G.A., Krashilina, A.V., Cherkasov, V.K. et al. Bis(1,4-di-tert-butyl-1,4-diazabutadiene)copper(i) [(3,6-di-tert-butyl-o-benzosemiquinono)(3,6-di-tert-butyl-catecholato)cuprate(ii)]. The molecular structure and intramolecular electron transfer. Russian Chemical Bulletin 50, 2193–2199 (2001). https://doi.org/10.1023/A:1015022006445
Issue Date:
DOI: https://doi.org/10.1023/A:1015022006445