Abstract
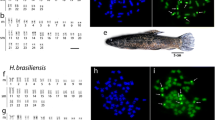
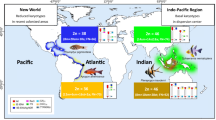
The karyotype of Chondrichthyes is still the least investigated among vertebrates. Over the last 40 years, the karyotypes of 63 out of the 1100 known species (5.73%) have been described in literature, namely seven squalomorph, one squatinomorph, 20 galeomorph, 33 batoid and two holocephalian species. Generally, the diploid number ranges from a minimum of 28 to a maximum of 106 elements, with more frequent values observed between 50 and 100 chromosomes. None of the four superorders is characterized by a peculiar chromosome set or morphology; the number of uniarmed and biarmed elements is variable in all the karyotypes, and microchromosomes are often present. The general trend in all groups seems to be a progressive reduction of the telocentric chromosome number in the most specialized species, followed by the loss of the microchromosomes. Polyploidy, followed by diploidization events and Robertsonian rearrangements, might have played a key role in the karyological evolution of elasmobranch fish. Chondrichthyes have the largest genome sizes among vertebrates, with the exception of dipnoans and urodeles. In the whole class, the species examined vary greatly in size, from 3 to 34 pg/N: the lowest values have been observed in holocephalians, while galeoids and batoids have a DNA amount ranging from 5 to 15 pg/N. Squaloids show heterogeneous DNA amounts, ranging from 8 to 34 pg/N. In more recent years, karyological studies have provided new data on the characterization of selachian karyotypes by C-banding, NOR staining, restriction enzymes in situ digestion and FISH with specific DNA probes, such as telomeric and SINE sequences.
Similar content being viewed by others
References
Amemiya, C.T. & J.R. Gold, 1988. Chromosomal NORs as taxonomic and systematic characters in North American cyprinid fish. Genetica 76: 81–90.
Asahida, T. & H. Ida, 1989. Karyological notes on four sharks in the order Carcharhiniformes. Jap. J. Ichthyol. 36: 275–280.
Asahida, T. & H. Ida, 1990. Karyotypes of two rays, Torpedo tokionis and Dasyatis matsubarai, and their systematic relationships. Jap. J. Ichthyol. 37: 71–75.
Asahida, T. & H. Ida, 1995. Karyotype and cellular DNA content of a guitarfish, Rhinobatos schlegelii. La Kromosomo II 79-80: 2725–2730.
Asahida, T., H. Ida & K. Hayashizaki, 1995. Karyotypes and cellular DNA contents of some sharks in the order Carcharhiniformes. Jap. J. Ichthyol. 42: 21–26.
Asahida, T., H. Ida & S. Inoue, 1987. Karyotypes of three rays in the order Myliobatiformes. Jap. J. Ichthyol. 33: 426–430.
Asahida, T., H. Ida & T. Inoue, 1988. Karyotypes and cellular DNA contents of two sharks in the family Scyliorhinidae. Jap. J. Ichthyol. 35: 215–219.
Asahida, T., H. Ida, H. Terashima & H.Y. Chang, 1993. The karyotype and cellular DNA content of a ray, Mobula japonica. Jap. J. Ichthyol. 40: 317–322.
Bernardi, G. & G. Bernardi, 1990. Compositional patterns in the nuclear genomes of cold-blooded vertebrates. J. Mol. Evol. 31: 265–281.
Cappetta, H., 1987. Chondrichthyes II: mesozoic and cenozoic elasmobranchii, pp 1–193 in Handbook of Paleoichthyology, Vol. 3B, edited by H.P. Schultze. Gustav Fischer Verlag, Stuttgart.
Cavalier-Smith, T. & M.J. Beaton, 1999. The skeletal function of non-genic nuclear DNA: new evidence from ancient cell chimaeras. Genetica 106: 3–13.
Cavalier-Smith, T., 1982. Skeletal DNA and the evolution of genome size. Ann. Rev. Biophys. Bioeng. 11: 273–302.
Chang, H.-Y., T.-K. Sang, K.-Y. Jan & C.-T. Chen, 1995. Cellular DNA contents and cell volumes of batoids. Copeia 3: 571–576.
Compagno, L.J.V., 1999. Systematics and body form, pp 1–42 in Sharks, Skates and Rays, edited by W.C. Hamlett. The Johns Hopkins University Press, Baltimore.
Compagno, L.J.V., 1973. Interrelationships of living Elasmobranchs, pp 15–61 in Interrelationships of Fishes, edited by P.H. Greenwood, R.S. Miles & C. Patterson. Academic Press, New York.
Compagno, L.J.V., 1977. Phyletic relationships of living sharks and rays. Ann. Zool. 17: 303–322.
Compagno, L.J.V., 1984. Sharks of the World. FAO Species Catalogue, United Nations Food and Agriculture Organization, Rome.
Compagno, L.J.V., 1988. Sharks of the Order Carcharhiniformes. Princeton University Press, Princeton.
De Carvalho, M.R., 1996. Higher-level elasmobranch phylogeny, basal squalians, and paraphyly, pp. 35–62 in Interrelationships of Fishes, edited by M.L.J. Stiassny, L.R. Parenti & G.D. Johnson. Academic Press, San Diego.
Donahue, W.H., 1974. A karyotypic study of three species of Rajiformes (Chondrichthyes, Pisces). Can. J. Genet. Cytol. 16: 203–211.
Garagna, S., C.A. Redi, E. Capanna, N. Andayani, R.M. Alfano, P. Doi & G. Viale, 1993. Genome distribution, chromosomal allocation, and organization of the major and minor satellite DNA in 11 species and subspecies of the genus Mus. Cytogenet. Cell Genet. 64: 247–255.
Gaudin T.J., 1991. A re-examination of elasmobranch monophyly and chondrichthyan phylogeny. N. Jahrb. Geol. Palaontol. Abhandl. 182: 133–160.
Goto, M., 1985. Evolution and adaptation of elasmobranch thooth. Rep. Japanese Group for Elasmobranch Studies 21: 28.
Hinegardner R.T., 1976. The cellular DNA content of sharks, rays and some other fishes. Comp. Biochem. Physiol. 55B: 367–370.
Ida, H., I. Sato & N. Miyawaki, 1985. Karyotypes of two species in the order Torpediniformes. Jap. J. Ichthyol. 32: 107–111.
Ida, H., T. Asahida, K. Yano & S. Tanaka, 1986. Karyotypes of two sharks, Chlamydoselachus anguineus and Heterodontus japonicus, and their systematic implications, pp. 158–163 in Indo Pacific Fish Biology, edited by T. Uyeno, R. Arai, T. Taniuchi & K. Matsuura. Ichthyol. Soc. Of Japan, Tokyo.
Kendall, C., S. Valentino, A.B. Bodine & C.A. Luer, 1992. Flow cytometric DNA analysis of nurse shark, Ginglymostoma cirratum (Bonaterre) and clearnose skate, Raja eglanteria (Bosc) peripheral red blood cells. J. Fish Biol. 41: 123–129.
Kendall, C., S. Valentino, A.B. Bodine & C.A. Luer, 1994. Triploidy in a nurse Shark, Ginglymostoma cirratum. Copeia 1994: 825–827.
Kikuno, T. & Y. Ojima, 1987. A Karyotypic studies of a guitar fish, Rhinobatos hyinnicephalus Richardson (Pisces, Rajiformes). La Kromosomo II-47–48: 1538–1544.
Lozano, R., C. Ruiz Rejòn & M. Ruiz Rejòn, 1991. An analysis of coho salmon chromatin by means of C-banding, Agand fluorochrome staining, and in situ digestion with restriction endonucleases. Heredity 66: 403–409.
Maddock, M.B. & F.J. Schwartz, 1996. Elasmobranch cytogenetics: methods and sex chromosomes. Bull. Mar. Sci. 58: 147–155.
Maisey, J.G. & K.E. Wolfram, 1984. Notidanus, pp. 170–180 in Living Fossils. Springer-Verlag, New York.
Maisey, J.G., 1982. The anatomy and interrelationships of Mesozoic Hybodont sharks. Amer. Mus. Novit. 2724: 1–48.
Maisey, J.G., 1989. Hamiltonichthys mapesi g. & sp. Nov. (Chondrichthyes; Elasmobranchii), from the Upper Pennsylvanian of Kansas. Am. Mus. Novit. 2931: 1–42.
Makino, S., 1937. The chromosomes of two elasmobranch fishes. Cytologia 2: 867–876.
Martin, A.P., G.J.P. Naylor & S.R. Palumbi, 1992. Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature 357: 153–155.
Martinez, P., A. Vinas, C. Bouza, J. Castro & L. Sanchez, 1991. Quantitative analysis of the variability of nucleolar organizer regions in Salmo trutta. Genome 36: 1119–1123.
Matthey, R., 1937. La formule chromosomiale du Selacien Scyliorhinus catula. C. R. Seanc. Soc. Biol. 126: 388–389.
McEachran, J.D., K.A. Dunn & T. Miyake, 1996. Interrelationships of the batoid fishes (Chondrichthyes: Batoidei), pp. 63–84 in Interrelationships of Fishes, edited by M.L.J. Stiassny, L.R. Parenti & G.D. Johnson. Academic Press, San Diego.
Mirsky, A.E. & H. Ris, 1951. The desoxyribonucleic acid content of animal cells and its evolutionary significance. J. Gen. Physiol. 34: 451–462.
Morescalchi, A., 1970. Karyology and vertebrate phylogeny. Boll. Zool. 37: 1–28.
Morescalchi, A., 1977. Phylogenetic aspects of karyological evidence, pp. 149–167 in Major Patterns in Vertebrate Evolution, edited by M.K. Hecht, P.C. Goody & B.M. Hecht. Pl. Publ. Corp., New York.
Nakaja, K., 1975. Taxonomy, comparative anatomy and phylogeny of Japanese catsharks, Scyliorhinidae. Mem. Fac. Fish. Hokkaido Univ. 23: 1–94.
Nishida, K., 1990. Phylogeny of the suborder Myliobatidoidei. Mem. Fac. Fish. Hokkaido U. 37: 1–108.
Nogusa, S., 1960. A comparative study of the chromosomes of fishes with particular considerations on taxonomy and evolution. Mem. Hyogo Univ. Agric. 3: 1–62.
Nygren, A. & M. Jahnke, 1972. Microchromosomes in primitive fishes. Swed. J. Agric. Res. 2: 229–238.
Nygren, A., B. Nilsson & M. Jahnke, 1971. Cytological study in Hypotremata and Pleurotremata (Pisces). Hereditas 59: 275–282.
Odierna, G., G. Aprea, O.J. Arribas, T. Capriglione, V. Caputo & E. Olmo, 1996. The karyology of the iberian rock lizards. Herpetologica 52: 542–550.
Ogiwara, I., M. Miya, K. Ohshima & N. Okada, 1999. Retropositional parasitism of SINEs on LINEs: identification of SINEs and LINEs in elasmobranchs. Mol. Biol. Evol. 16: 1238–1250.
Ohno, S., 1970. Evolution by Gene Duplication. Springer Verlag, New York.
Ohno, S., J. Muramoto, C. Stenius, L. Christian, W.A. Kittrel & N.B. Atkin, 1969. Microchromosomes in holocephalian, chondrostean and holostean fishes. Chromosoma 26: 35–40.
Olmo, E., V. Stingo, G. Odierna & T. Capriglione, 1982b. Sequence organization in the DNA of three selachians. Experientia 38: 339–340.
Olmo, E., V. Stingo, O. Cobror, T. Capriglione & G. Odierna, 1982a. Repetitive DNA and polyploidy in selachians. Comp. Biochem. Physiol. B 73: 739–745.
Ozouf-Costaz, C., E. Pisano, C. Bonilio & R. Williams, 1996. Ribosomal RNA location in the Antarctic fish Champsocephalus gunnati (Notothenioidei, Channichthyidae) using banding and fluorescence in situ hybridization. Chromosome Res. 4:557–561.
Pardini, A.T., C.S. Jones, M.C. School & L.R. Noble, 2000. Isolation and characterization of dinucleotide microsatellite loci in the Great White Shark, Carcharodon carcharias. Mol. Ecol. 9: 1176–1178.
Pedersen, R.A., 1971. DNA content ribosomal gene multiplicity and cell size in fish. J. Exp. Zool. 177: 65–78.
Phillips, R.B., K.A. Pleyte & P.E. Ihssen, 1989. Patterns of chromosomal nucleolar organizer region (NOR) variation in fishes of the genus Salvelinus. Copeia 1989: 47–53.
Rocco, L., V. Stingo & M. Bellitti, 1996. Cloning and characterization of a repetitive DNA detected by Hind III in the genome of Raja montagui (Batoidea, Chondrichthyes). Gene 176: 185–189.
Rodrigues, E. & M.J. Collares-Pereira, 1996. NOR polymorphism in the Iberian species Chondrostoma lusitanicum (Pisces: Cyprinidae). Genetica 98: 59–63.
Schmid, M., 1982. Chromosome banding in Amphibia. XII. Analysis of the structure and variability of NORs in Anura. Chromosoma 87: 327–344.
Schwartz, F.J. & M.B. Maddock, 1986. Comparisons of karyotypes and cellular DNA contents within and between major lines of elasmobranch p. 148 in Indo-Pacific Fish Biology, edited by T. Uyeno, R. Arai, T. Tuniuchi and K. Matsuura. Ichthyological Society Japan, Tokyo.
Shimoda, N., M. Chevrette, M. Ekker, J. Kikuchi, Y. Hotta & H. Okamoto, 1996. Mermaid: a family of short interspersed repetitive elements widespread in vertebrates. Biochem. Biophysic. Res. Comm. 220: 226–232.
Shirai, S., 1996. Phylogenetic interrelationships of neoselachians (Chondrichthyes, Euselachii), pp. 9–34 in Interrelationships of Fishes, edited by M.L.J. Stiassny, L.R. Parenti & G.D. Johnson. Academic Press, San Diego.
Sidow, A., 1996. Gen(om)e duplications in the evolution of early vertebrates. Curr. Opin. Genet. Dev. 6: 715–722. Review.
Stingo, V. & L. Rocco, 1991. Chondrichthyan cytogenetics: a comparison with Teleosteans. J. Mol. Evol. 33: 76–82.
Stingo, V. & T. Capriglione, 1986. DNA and chromosomal evolution in cartilaginous fish, pp. 140–147 in Indo-Pacific Fish Biology, edited by T. Ueno, R. Arai, T. Taniuchi & K. Matsuura. Ichthyological Society Japan, Tokyo.
Stingo, V., 1976. Cariologia di due torpedini italiane. Boll. Zool. 43: 406–407.
Stingo, V., 1978. Ulteriori dati cariologici sui selaci italiani. Boll. Zool. 45: 243.
Stingo, V., 1979. New developments in vertebrate citotaxonomy. II. The chromosomes of the cartilaginous fishes. Genetica 50: 227–239.
Stingo, V., L. Rocco & R. Improta, 1989b. Chromosome markers and karyology of Selachians. J. Exp. Zool. suppl 2: 175–185.
Stingo, V., L. Rocco, G. Odierna & M. Bellitti, 1995. NOR and heterochromatin analysis in two cartilaginous fishes by C-, Ag-and RE (restriction endonuclease)-banding. Cytogenet. Cell Genet. 71: 228–234.
Stingo, V., M.H. Du Buit & G. Odierna, 1980. Genome size of some selachian fishes. Boll. Zool. 47: 129–137.
Stingo, V., T. Capriglione, L. Rocco, R. Improta & A. Morescalchi, 1989a. Genome size and A-T rich DNA in Selachians. Genetica 79: 197–205.
Szarski, H., 1976. Cell size and nuclear DNA content in Vertebrates. Int. Rev. Cytol. 44: 93–111.
Vialli, M., 1957. Volume et contenu en ADN par noyau, in Cytochemical methods with quantitative aims. Exp. Cell Res. (4suppl.): 284–293.
White, M.J.D., 1973. Animal cytology and evolution. Cambridge Univ. Press, London, 3rd edn.
Yabu, H. & K. Ishii, 1984. Chromosomes of the great blue shark Prionace glauca (Linnaeus). Bull. Japan. Soc. Sci. Fish. 50: 7–10.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stingo, V., Rocco, L. Selachian cytogenetics: a review. Genetica 111, 329–347 (2001). https://doi.org/10.1023/A:1013747215866
Issue Date:
DOI: https://doi.org/10.1023/A:1013747215866