Abstract
A growing number of physiological and pathophysiological processes have been shown to be influenced by leptin apart from its first recognised role as a modulator of hypothalamic appetite and weight control centers. We investigated the presence and pattern of distribution of leptin mRNA and the mRNA of the long isoform of the leptin receptor in the normal pituitary and in different types of pituitary adenomas. We also studied leptin secretion from human pituitary tumors in culture, and the in vitro pituitary hormone release following stimulation with human leptin. Leptin mRNA expression was detected at a low level of expression in 50% of tumors but in none of the normal pituitaries. By immunohistochemistry, leptin was present in occasional scattered cells in the normal pituitary and in pituitary tumors. The leptin receptor long isoform was detected in the majority (65%) of pituitary tumors and in all normal pituitaries. It did not segregate with any particular tumor type, and varying levels of expression were detected between the tissues studied. 34% of pituitary adenomas showed leptin release into the incubation media during in vitro culture. Leptin mRNA, the mRNA of the long isoform of the receptor, or in vitro leptin release, did not correlate with tumor type or with any of the other pituitary hormones released. In vitro leptin stimulation of pituitary tumors caused stimulation of FSH and α-subunit secretion from a non-functioning adenoma and TSH secretion from a somatotroph adenoma. As the co-localisation of ACTH and leptin in corticotroph cells was previously suggested, we investigated whether in vivo ACTH release is accompanied by a simultaneous plasma leptin level rise (i) in peripheral plasma samples after food intake-induced ACTH rise in healthy obese and nonobese individuals and (ii) in petrosal sinus samples after CRH injection in Cushing's disease patients. Our data suggest that a rise in ACTH levels is not accompanied by detectable rise in leptin levels in peripheral and in petrosal sinus blood samples. In summary, leptin is synthesized and stored within the pituitary and may modulate other pituitary hormone secretion, although probably do not contribute to plasma leptin level changes. Pituitary leptin may therefore be a novel paracrine regulator of pituitary function.
Similar content being viewed by others
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432.
Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat Med 1997;3:1029–1033.
Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature 1998;394:790–793.
Wang JL, Liu R, Hawkins M, Barzilai N, Rossetti L. Anutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998;393:684–688.
Heiman ML, Chen Y, Caro JF. Leptin participates in the regulation of glucocorticoid and growth hormone axes. J Nutr Biochem 1998;9:553–559.
Mantzoros CS, Moschos SJ. Leptin: In search of role(s) in human physiology and pathophysiology. Clin Endocrinol (Oxf) 1998;49:551–567.
Gainsford T, Wilson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation and functional activation of hematopoetic cells. Proc Natl Acad Sci USA 1996;93:14564–14568.
Shimabukuro M, Koyama K, Chen GX, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA 1997;94:4637–4641.
Lord GM, Matarase G, Howard LK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897–901.
Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science 1998; 281:1683–1686.
Myers DA, Bell ME, McDonald TJ, Myers TR. Corticotropin-releasing factor receptor expression in the pituitary of fetal sheep after lesion of the hypothalamic paraventricular nucleus. Endocrinology 1999;140:4292–4299.
Couce ME, Burguera B, Parisi JE, Jensen MD, Lloyd RV. Localization of leptin receptor in the human brain. Neuroendocrinology 1997;66:145–150.
Burguera B, Couce ME, Long J, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology 2000;71:187–195.
Lee GH, Proenca R, Montez JM, et al.Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379: 632–635.
Shimon I, Yan XM, Magoffin DA, Friedman TC, Melmed S. Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. J Clin Endocrinol Metab 1998;83:4059–4064.
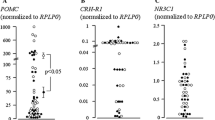
Jin L, Burguera BG, Couce ME, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence for a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab 1999;84: 2903–2911.
Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland.Endocrinology 1999;140:5995–5998.
Iqbal J, Pompolo S, Considine RV, Clarke IJ. Localisation of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology 2000;141:1515—1520.
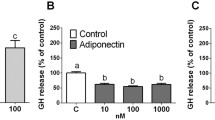
Korbonits M, Chitnis MM, Norman D, et al. The release of leptin and its effect on hormone release from human pituitary adenomas. Clin Endocrinol (Oxf) 2001; (In Press)
Jin L, Zhang S, Burguera BG, et al.Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 2000;141:333–339.
Vidal S, Cohen SM, Horvath E, et al. Subcellular localization of leptin in non-tumorous and adenomatous human pituitaries: an immuno-ultrastructural study. J Histochem Cytochem 2000;48:1147–1152.
Wiesner G, Vaz M, Collier G, et al. Leptin is released from the human brain: Influence of adiposity and gender.J Clin Endocrinol Metab 1999;84:2270–2274.
Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA 1997;94:1023–1028.
Korbonits M, Trainer PJ, Nelson ML, et al. Differential stimulation of cortisol and dehydroepiandrosterone levels by food in obese and normal subjects: Relation to body fat distribution. Clin Endocrinol (Oxf) 1996;45:699–706.
Korbonits M, Trainer PJ, Little JA, et al. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary adrenal activity. Clin Endocrinol (Oxf) 1997;46:751–757.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korbonitz, M., Chitnis, M.M., Gueorguiev, M. et al. Leptin in Pituitary Adenomas–A Novel Paracrine. Pituitary 4, 49–56 (2001). https://doi.org/10.1023/A:1012934710471
Issue Date:
DOI: https://doi.org/10.1023/A:1012934710471