Abstract
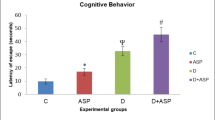
We studied a protective effect of a course injections of melatonin on cognitive deficiency in rats with streptozotocin-induced diabetes (STZD). The mean time necessary for the fulfillment of the Morris' water test in animals with STZD after 7 days of testing was three times greater than the corresponding index in the control group. Rats with STZD, which were injected with 10 mg/kg melatonin daily for 21 days after introduction of STZ, demonstrated a significantly lower level of cognitive deficiency ((in these rats the mean time necessary for the test fulfillment was only 48% greater than that in the control animals). In rats with STZD, substantial changes in the content of NCAM isoforms in the brain structures (significant decreases in the NCAM180 content in the hippocampus, neocortex, and cerebellum, and in that of NCAM140 in the cerebellum) were observed. Course injections of melatonin into the rats with STZD promoted significant normalization of the composition of NCAM isoforms in the structures under study. The data obtained indicate that control of expression of separate NCAM isoforms can be one of the mechanisms through which melatonin prevents the development of cognitive deficiency in diabetic animals.
Similar content being viewed by others
REFERENCES
W. H. Gispen and G.-J. Biessels, “Cognition and synaptic plasticity in diabetes mellitus, ” Trends Neurosci., 23, 542–549 (2000).
M. A. Smith, “Radical ageing in Alzheimer's disease, ” ?rends Neurosci., 18, 172–176 (1995).
J. Prockaerts, T. Fahrig, and A. Blokland, “Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an i.c.v. injection of streptozotocin: a correlation analysis, ” Behav.Brain Res., 102, 73–88 (1999).
J. T. Coyle and P. Puttfarcken, “Oxidative stress, glutamate, and neurodegenerative disorders, ” Science, 262, 689–695 (1993).
R. J. Reiter, D. Acuna-Castroviejo, D. X. Tan, and S. Burkhardt, “Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in central nervous system, ” Ann.New York Acad.Sci., 939, 200–215 (2001).
M. Sharma and Y. K. Gupta, “Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment, ” Life Sci., 68, 1021–1029 (2001).
O. O. Litvin and K.V. Anokhin, “Mechanisms of memory reorganization during retrieval of acquired behavioral experience in chicks: the effects of protein synthesis inhibition in the brain, ” Neurosci.Behav.Physiol., 30, No. 6, 671–678 (2000).
C. E. Teunissen, H. W. Steinbusch, M. Angevaren, et al., “Behavioural correlates of striatal glial fibrillary acidic protein in the 3-nitropropionic acid rat model: disturbed walking pattern and spatial orientation, ” Neuroscience, 105, No. 1, 153–167 (2001).
G. Baydas, V. S. Nedzvetsky, P. A. Nerush, et al., “A novel role for melatonin: regulation of the expression of cell adhesion molecules in the rat hippocampus and cortex, ” Neurosci.Lett., 326, No. 2, 109–112 (2002).
O. H. Laemmli, “Cleavage of structural proteins during the assembly of the head of bacteriophage T4, ” Nature, 227, No. 1, 243–246 (1970).
V. S. Nedzvetsky, G. Baydas, P. A. Nerush, et al., “Melatonin is involved in regulation of the expression of neural cell adhesion molecules in the rat brain, ” Neirofiziologiya/Neurophysiology, 34, Nos. 2/3, 204–207 (2002).
G. L. Miller, “Protein determination for large numbers of samples, ” Anal.Chem., 31, No. 5, 964–966 (1959).
D. A. Greene, “Sorbitol, phosphoinositides, and sodiumpotassium-ATPase in the pathogenesis of diabetic complications, ” New Engl.J.Med., 316, 599–606 (1987).
G. Baydas, H. Canatan, and A. Turkoglu, “Comparative analysis of the protective effects of melatonin and vitamin E on streptozotocin-induced diabetes mellitus, ” J. Pineal Res., 32, 225–230 (2002).
R. J. Reiter, D. Acuna-Castroviejo, D. Z. Tan, et al., “Free radical-mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system, ” Ann.New York Acad.Sci., 939, 200–215 (2001).
M. J. Forster, A. Dubey, K. M. Dawson, et al., “Agerelated losses of cognitive function and motor skills in mice ?r? associated with oxidative protein damage in the brain, ” ?ro?.Natl.Acad.Sci.USA, 9?, 4765–4769 (1996).
R. J. Reiter, “Oxidative damage in the central nervous system: protection by melatonin, ” Prog.Neurobiol., 56, 359–384 (1998).
M. V. Hogan, Y. El-Sherif, and A. Wieraszko, “The modulation of neuronal activity by melatonin: in vitro studies on mouse hippocampal slices, ” J.Pineal Res., 30, No. 2, 87–96 (2001).
S. Y. Shiu, L. Li, S. W. Siu, et al., “Biological basis and possible physiological implications of melatonin receptormediated signaling in the rat epididymis, ” Biol. Signals Recept., 9, Nos. 3/4, 172–187 (2000).
M. Amoureux, B. A. Cunningham, G. M. Edelman, et al., “NCAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype, ” J.Neurosci., 20, No. 10, 3631–3640 (2000).
L. C. Ronn, V. Berezin, and E. Bock, “The neural cell adhesion molecule in synaptic plasticity and ageing, ” Int.J.Dev.Neurosci., 18, 193–199 (2000).
H. Shen, M. Watanabe, H. Tomasiewicz, et al., “Genetic deletions of NCAM and PSA impair circadian function in the mouse, ” Physiol.Behav., 73, Nos. 1/2, 185–193 (2001).
J. Z. Kiss, “A role of adhesion molecules in neuronal plasticity, ” Mol.Cell.Endocrinol., 140, 89–94 (1998).
G. G. Scibo, H. A. Davies, D. A. Rusakiv, et al., “Increased immunogold labelling of neural cell adhesion molecule isoform in synaptic active zones of the chick striatum 5–6 hours after one-trial passive avoidance training, ” Neuroscience, 82, No. 1, 1–5 (1998).
H. Cremer, R. Lange, A. Christoph, et al., “Inactivation of the NCAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning, ” Nature, 367, 455–459 (1994).
T. Schuster, M. Krug, H. Hassan, et al., “Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation, ” J.Neurobiol., 37, No. 3, 359–372 (1998).
R. Minana, M. Sancho-Tello, E. Climent, et al., “Intracellular location, temporal expression, and polysialylation of neural cell adhesion molecule in astrocytes in primary culture, ” Glia, 24, No. 4, 415–427 (1998).
M. Fujimoto, J. L. Bruses, and U. Rutishauser, “Regulation of cell adhesion by polysialic acid, ” J.Biol.Chem., 276, No. 34, 31745–31751 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nedzvetsky, V.S., Nerush, P.A. & Kirichenko, S.V. Effect of Melatonin on Cognitive Ability of Rats and Expression of NCAM in the Brain Structures in Streptozotocin-Induced Diabetes. Neurophysiology 35, 422–427 (2003). https://doi.org/10.1023/B:NEPH.0000024603.71459.f9
Issue Date:
DOI: https://doi.org/10.1023/B:NEPH.0000024603.71459.f9