Abstract
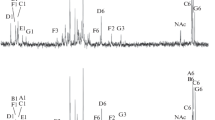
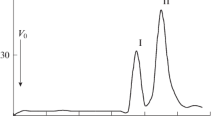
A comparative study of the lipopolysaccharides (LPS) isolated from Sinorhizobium meliloti SKHM1-188 and two of its LPS mutants (Tb29 and Ts22) with sharply decreased nodulation competitiveness was conducted. Polyacrylamide gel electrophoresis with sodium dodecyl sulfate revealed two forms of LPS in all three strains: a higher molecular weight LPS1, containing O-polysaccharide (O-PS), and a lower molecular weight LPS2, without O-PS. However, the LPS1 content in mutants was significantly smaller than in the parent strain. The LPS of the strains studied contained glucose, galactose, mannose, xylose, three nonidentified sugars (X 1 (TGlc 0.53), X 2 (TGlc 0.47), and X 3 (TGlc 0.43)), glucosamine, and ethanolamine, while the LPS of S. meliloti SKHM1-188 additionally contained galactosamine, glucuronic and galacturonic acids, and 2-keto-3-deoxyoctulosonic acid (KDO), as well as such fatty acids as 3-OH C14:0, 3-OH C15:0, 3-OH C16:0, 3-OH C18:0, nonidentified hydroxy X (T3-OH C14:0 1.33), C18:0, and unsaturated C18:1 fatty acids. The LPS of both mutants were similar in the component composition but differed from the LPS of the parent strain by lower X 2, X 3, and 3-OH C14:0 contents and higher KDO, C18:0, and hydroxy X contents. The LPS of all the strains were subjected to mild hydrolysis with 1% acetic acid and fractionated on a column with Sephadex G-25. The higher molecular weight fractions (2500–4000 Da) contained a set of sugars typical of intact LPS and, supposedly, corresponded to the LPS polysaccharide portion (PS1). In the lower molecular weight fractions (600–770 Da, PS2), glucose and uronic acids were the major components; galactose, mannose, and X 1 were present in smaller amounts. The PS1/PS2 ratio for the two mutants was significantly lower than for strain SKHM1-188. The data obtained show that the amount of O-PS–containing molecules (LPS1) in the heterogeneous lipopolysaccharide complex of the mutants was smaller than in the SKHM1-188 LPS; this increases the hydrophobicity of the cell surface of the mutant bacteria, which supposedly contributes to their nonspecific adhesion to the roots of the host plant, thus decreasing their nodulation competitiveness.
Similar content being viewed by others
REFERENCES
Kannenberg, E.L., Reuhs, B.L., Forsberg, S.L., and Carlson, R.W., Lipopolysaccharides and K-Antigens: Their Structures, Biosynthesis, and Functions, The Rhizobiaceae, Spaink, H.H. et al., Eds., Kluwer, 1998, pp. 119-154.
Lagares, A., Caetano-Annoles, G., Nichaus, K., et al., A Rhizobium meliloti Lipopolysaccharide Mutant Altered in Competitiveness for Nodulation of Alfalfa, J. Bacteriol., 1992, vol. 174,no. 8, pp. 5941-5952.
Araujo, R.S., Robleto, E.A., and Handelsman, J.O., A Hydrophobic Mutant of Rhizobium etli Altered in Nodulation Competitiveness and Growth in the Rhizosphere, Appl. Environ. Microbiol., 1994, vol. 60,no. 5, pp. 1430-1436.
Zatovskaya, T.V., Ushakov, K.V., Yurgel', S.N., Kosenko, L.V., Zakharova, I.Ya., and Simarov, B.V., Obtaining of Tn5 Mutants of Rhizobium meliloti with Altered Composition of Lipopolysaccharides and Analysis of Their Symbiotic Properties, Genetika, 1998, vol. 34,no. 6, pp. 742-748.
Zatovskaya, T.V., Kosenko, L.V., Yurgel', S.N., and Simarov, B.V., LPS Mutants of Sinorhizobium meliloti and Their Nodulation Competitiveness, Mikrobiol. Zh., 2000, vol. 62,no. 2, pp. 27-37.
Westphal, O. and Jann, K., Bacterial Lipopolysaccharides. Extraction with Phenol-Water and Further Applications of the Procedure, Methods Carbohydr. Chem., 1965, vol. 5, pp. 83-91.
Laemmli, U.K., Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4, Nature, 1970, vol. 227, pp. 680-683.
Determan, G.G., Gel'-khromatografiya (Gel Chromatography), Moscow: Mir, 1970.
Moss, C.W., Samuels, S.B., Liddie, J., and McKinney, R.M., Occurrence of Branched-Chain Hydroxyl Fatty Acids in Pseudomonas maltophylia, J. Bacteriol., 1973, vol. 114,no. 3, pp. 1018-1020.
Zakharova, I.A. and Kosenko, L.V., Metody izucheniya mikrobnykh polisakharidov (Methods for Studying Microbial Polysaccharides), Kiev: Naukova Dumka, 1982.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F., Colorimetric Method for Determination of Sugars and Related Substances, Anal. Chem., 1956, vol. 28,no. 3, pp. 350-356.
Lowry, O.H., Rosebrough, N.J., Farr, A.K., and Randall, R.J., Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem., 1951, vol. 153,no. 1, pp. 265-275.
Bhat, U.R., Mayer, H., Yokota, A., Hollingsworth, R.I., and Carlson, R.W., Occurrence of Lipid A Variants with 27-Hydroxyoctacosanoic Acid in Lipopolysaccharides from Members of Family Rhizobiaceae, J. Bacteriol., 1991, vol. 173,no. 7, pp. 2155-2159.
Urbanik-Supniewska, T., Seydel, U., Greek, M., Weckesser, J., and Mayer, H., Chemical Studies on the Lipopolysaccharide of Rhizobium meliloti 10405 and Its Lipid A Region, Arch. Microbiol., 1989, vol. 152,no. 6, pp. 527-532.
Russa, R., Bruneteau, M., Shashkov, A.S., Urbanik-Sypniewska, T., and Mayer, H., Characterization of the Lipopolysaccharides from Rhizobium meliloti Strain 102F51 and Its Nonnodulating Mutant WL113, Arch. Microbiol., 1996, vol. 165, pp. 26-33.
Campbell, G.R.O., Reuhs, B.L., and Walker, G.C., Chronic Intracellular Infection of Alfalfa Nodules by Sinorhizobium meliloti Requires Correct Lipopolysaccharide Core, Proc. Natl. Acad. Sci. USA, vol. 99,no. 6, pp. 3938-3943.
Carlson, R.W., Heterogeneity of Rhizobium Lipopolysaccharides, J. Bacteriol., 1984, vol. 158,no. 3, pp. 1012-1017.
Carlson, R.W., Kalembasa, S., Turowski, D., Pachori, P., and Noel, K.D., Characterization of the Lipopolysaccharide from a Rhizobium phaseoli Mutant That Is Defective in Infection Thread Development, J. Bacteriol., 1987, vol. 169,no. 11, pp. 4923-4928.
Kosenko, L.V. and Zatovska, T.V., Interconnection of Biochemical and Functional Properties of Lipopolysaccharides from Rhizobium leguminosarum bv. viciae, Curr. Plant. Sci. Biotech. Agric., 1995, vol. 27, p. 698.
Rietschel, E.T., Brade, H., Holst, O., Brade, Z., Muller-Zoennies, S., Mamat, U., Zahringer, U., Beckmann, F., Seydel, U., Brandenburg, K., et al., Bacterial Endotoxin: Chemical Constitution, Biological Recognition, Host Response and Immunological Detoxification, Curr. Top. Microbiol. Immunol., 1996, vol. 216, pp. 39-81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kosenko, L.V., Zatovskaya, T.V. Investigation of Lipopolysaccharides from Sinorhizobium meliloti SKHM1-188 and Two of Its Mutants with Decreased Nodulation Competitiveness. Microbiology 73, 292–299 (2004). https://doi.org/10.1023/B:MICI.0000032239.51884.52
Issue Date:
DOI: https://doi.org/10.1023/B:MICI.0000032239.51884.52