Abstract
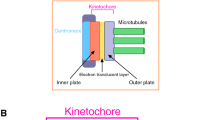
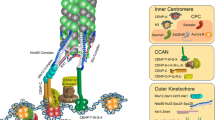
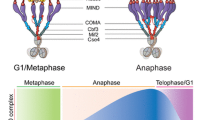
Proper segregation of chromosomes during cell division is essential for the maintenance of genetic stability. During this process chromosomes must establish stable functional interactions with microtubules through the kinetochore, a specialized protein structure located on the surface of the centromeric heterochromatin. Stable attachment of kinetochores to a number of microtubules results in the formation of a kinetochore fibre that mediates chromosome movement. How the kinetochore fibre is formed and how chromosome motion is produced and regulated remain major questions in cell biology. Here we look at some of the history of research devoted to the study of kinetochore–microtubule interaction and attempt to identify significant advances in the knowledge of the basic processes. Ultrastructural work has provided substantial insights into the structure of the kinetochore and associated microtubules during different stages of mitosis. Also, recent in-vivo studies have probed deep into the dynamics of kinetochore-attached microtubules suggesting possible models for the way in which kinetochores harness the capacity of microtubules to do work and turn it into chromosome motion. Much of the research in recent years suggests that indeed multiple mechanisms are involved in both formation of the k-fibre and chromosome motion. Thus, rather than moving to a unified theory, it has become apparent that most cell types have the capacity to build the spindle using multiple and probably redundant mechanisms.
Similar content being viewed by others
References
Agar WE (1912) The spermatogenesis of Lepidosiren paradoxa. Q J Micro Sci 57: 1–39.
Antonio C, Ferby I, Wilhelm H et al. (2000) Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102: 425–435.
Arnaoutov A, Dasso M (2003) The Ran GTPase regulates kinetochore function. Dev Cell 5: 99–111.
Bajer AS, Molè-Bajer J (1972) Spindle dynamics and chromosome movement. Int Rev Cytol Suppl. 3: 1–271.Baker JR (1951) Remarks on the discovery ofcell-division. ISIS 42: 285–287.
Bloom K (1993) The centromere frontier: kinetochore components, microtubule-based motility, and the CEN-value paradox. Cell 73: 621–624.
Bornens M (2002) Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol 14: 25–34.
Brenner S, Pepper D, Berns MW, Tan E, Brinkley BR (1981) Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol 91: 95–102.
Brinkley BR, Stubblefield E (1966) The fine structure ofthe kinetochore ofa mammalian cell in vitro. Chromosoma 19: 28–43.
Brinkley BR, Stubblefield E (1970) Ultrastructure and interaction ofthe kinetochore and the centriole in mitosis and meiosis. Adv Cell Biol 1: 119–185.
Brinkley BR, Valdivia MM, Tousson A, Brenner SL (1984) Compound kinetochores ofthe Indian muntjac. Evolution by linear fusion of unit kinetochores. Chromosoma 91: 1–11.
Brinkley BR, Zinkowski RP, Mollon WL et al. (1988) Movement and segregation ofkinetocho res experimentally detached from mammalian chromosomes. Nature 336: 251–254.
Carvalho P, Tirnauer JS, Pellman D (2003) Surfing on microtubule ends. Trends Cell Biol 13: 229–237.
Cassimeris L, Pryer NK, Salmon ED (1988) Real-time observations ofmicrotubule dynamic instability in living cells. J Cell Biol 107: 2223–2231.
Cassimeris L, Rieder CL, Salmon ED (1994) Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci 107 (Pt 1): 285–297.
Church K, Nicklas RB, Lin HP (1986) Micromanipulated bivalents can trigger mini-spindle formation in Drosophila melanogaster spermatocyte cytoplasm. J Cell Biol 103: 2765–2773.
Compton DA (2000) Spindle assembly in animal cells. Annu Rev Biochem 69: 95–114.
Corces VG, Salas J, Salas ML, Avila J (1978) Binding of microtubule proteins to DNA: specificity ofthe interaction.Eur J Biochem 86: 473–479.
Czaban BB, Forer A (1985a) The kinetic polarities ofspindle microtubules in vivo, in crane-fly spermatocytes. I. Kinetochore microtubules that re-form after treatment with colcemid. J Cell Sci 79: 1–37.
Czaban BB, Forer A (1985b) The kinetic polarities ofspindle microtubules in vivo, in crane-fly spermatocytes. II. Kinetochore microtubules in non-treated spindles. J Cell Sci 79: 39–65.
Darlington CD (1937) Recent Advances in Cytology, 2nd edn. Blakiston: Philadelphia.
De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J (1980) The microtubule nucleating and organizing capacity ofkinetocho res and centrosomes in living PTK2-cells. In: Microtubules and Microtubule InhibitorsDe Brabander M, J. De Mey, eds. Elsevier, Amsterdam, pp 255–268.
De Brabander M, Geuens G, De Mey J, Joniau M (1981) Nucleated assembly ofmitotic microtubules in living PTK2 cells after release from nocodazole treatment. Cell Motil 1: 469–483.
De Wulf P, McAinsh AD, Sorger PK (2003) Hierarchical assembly ofthe budding yeast kinetochore from multiple subcomplexes. Genes Dev 17: 2902–2921.
Debec A, Szollozi A, Szollozi D (1982) A Drosophila melanogaster cell line lacking centriole. Biol Cell 44: 133–138.
Ding R, McDonald KL, McIntosh JR (1993) Threedimensional reconstruction and analysis ofmitotic spindles from the yeast, Schizosaccharomyces pombe. J Cell Biol 120: 141–151.
Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, De Mey JR (1998) Evidence for a role of CLIP-170 in the establishment ofmetaphas e chromosome alignment. J Cell Biol 141: 849–862.
Euteneuer U, Ris H, Borisy GG (1983) Polarity ofkinetocho re microtubules in Chinese hamster ovary cells after recovery from a colcemid block. J Cell Biol 97: 202–208.
Flemming W (1965) Historical paper. Contributions to the knowledge ofthe cell and its vital processes. J Cell Biol 25: Suppl: 1-69.
Gorbsky GJ, Sammak PJ, Borisy GG (1987) Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J Cell Biol 104: 9–18.
Gould RR, Borisy GG (1978) Quantitative initiation of microtubule assembly by chromosomes from Chinese hamster ovary cells. Exp Cell Res 113: 369–374.
Hayden JH, Bowser SS, Rieder CL (1990) Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol 111: 1039–1045.
Heald R, Weis K (2000) Spindles get the ran around. Trends Cell Biol 10: 1–4.
Heald R, Tournebize R, Blank T et al. (1996) Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382: 420–425.
Hill TL (1985) Theoretical problems related to the attachment ofmicrotub ules to kinetochores. Proc Natl Acad Sci USA 82: 4404–4408. Jokelainen PT (1967) The ultrastructure and spatial organization ofthe metaphase kinetochore in mitotic rat cells.J Ultrastruct Res 19: 19–44
Jokelainen PT (1967) The ultrastructure and spatial organization ofthe metaphase kinetochore in mitotic rat cells.J Ultrastruct Res 19: 19–44
Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS (2001) A role for the adenomatous polyposis coli protein in chromosome segregation. Natl Cell Biol 3: 429–432.
Karsenti E, Kobayashi S, Mitchison T, Kirschner M (1984a) Role ofthe centrosome in organizing the interphase microtubule array: properties ofcytoplasts containing or lacking centrosomes. J Cell Biol 98: 1763–1776.
Karsenti E, Newport J, Kirschner M (1984b) Respective roles ofcentroso mes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J Cell Biol 99: 47s–54s.
Khodjakov A, Cole RW, Oakley BR, Rieder CL (2000) Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 10: 59–67.
Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM (2003) Minus-end capture ofpref ormed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol 160: 671–683.
King JM, Hays TS, Nicklas RB (2000) Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol 151: 739–748.
Kirschner M, Mitchison T (1986) Beyond self-assembly: from microtubules to morphogenesis. Cell 45: 329–342.
Lombillo VA, Nislow C, Yen TJ, Gelfand VI, McIntosh JR (1995) Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol 128: 107–115.
Maddox P, Straight A, Coughlin P, Mitchison TJ, Salmon ED (2003) Direct observation ofmicrotubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J Cell Biol 162: 377–382.
Maiato H, Sampaio P, Lemos CL et al. (2002) MAST/Orbit has a role in microtubule-kinetochore attachment and is essential for chromosome alignment and maintenance of spindle bipolarity}. J Cell Biol 157: 749–760.
Maiato H, Fairley EA, Rieder CL, Swedlow JR, Sunkel CE, Earnshaw WC (2003a) Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113: 891–904.
Maiato H, Rieder CL, Earnshaw WC, Sunkel CE (2003b) How do kinetochores CLASP dynamic microtubules? Cell Cycle 2: 511–514.
McEwen BF, Arena JT, Frank J, Rieder CL (1993) Structure ofthe colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol 120: 301–312.
McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL (1997) Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J Cell Biol 137: 1567–1580.
McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ (2001) CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell 12: 2776–2789.
Mitchison TJ (1989) Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol 109: 637–652.
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312: 237–242.
Mitchison TJ, Salmon ED (1992) Poleward kinetochore fibre movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol 119: 569–582.
Mitchison TJ, Salmon ED (2001) Mitosis: a history ofdivision. Nat Cell Biol 3: E17–21.
Mitchison TJ, Kirschner MW (1985) Properties ofthe kinetochore in vitro. II. Microtubule capture and ATPdependent translocation. J Cell Biol 101: 766–777.
Mitchison T, Evans L, Schulze E, Kirschner M (1986) Sites of microtubule assembly and disassembly in the mitotic spindle. Cell 45: 515–527.
Mole-Bajer J (1969) Fine structural studies ofapolar mitosis. Chromosoma 26: 427–448.
Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM (1980) Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci USA 77: 1627–1631.
Musacchio A, Hardwick KG (2002) The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol 3: 731–741.
Nicklas RB (1989) The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol 109: 2245–2255.
Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR (1990) Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 345: 263–265.
Pickett-Heaps JD (1969) The evolution ofthe mitotic apparatus: an attempt at comparative ultrastructural cytology in dividing cells. Cytobios 3: 257–280.
Pickett-Heaps JD, Tippit DH (1978) The diatom spindle in perspective. Cell 14: 455–467.
Pickett-Heaps JD, Tippit DH, Porter KR (1982) Rethinking mitosis. Cell 29: 729–744.
Putkey FR, Cramer T, Morphew MK et al. (2002) Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell 3: 351–365.
Rieder CL (1982) The formation, structure, and composition ofthe mammalian kinetochore and kinetochore fiber. Int Rev Cytol 79: 1–58.
Rieder CL, Alexander SP (1990) Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 110: 81–95.
Rogers SL, Rogers GC, Sharp DJ, Vale RD (2002) Drosophila EB1 is important for proper assembly, dynamics, and positioning ofthe mitotic spindle. J Cell Biol 158: 873–884.
Rogers GC, Rogers SL, Schwimmer TA et al. (2004) Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427: 364–370.
Roos UP (1973) Light and electron microscopy ofrat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma 41: 195–220. Savoian MS, Goldberg ML, Rieder CL (2000) The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat Cell Biol 2: 948–
Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ (1997) CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol 139: 1373–1382.
Schrader F (1944) Mitosis. The Movement of Chromosomes in Cell Division. New York: Columbia University Press.
Schuyler SC, Pellman D (2001). Microtubule 'plus-endtracking proteins': The end is just the beginning. Cell 105: 421–424.
Sharp LW (1934) Introduction to Cytology. New York and London: McGraw-Hill.
Sharp DJ, Rogers GC, Scholey JM (2000) Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat Cell Biol 2: 922–930.
Skibbens RV, Skeen VP, Salmon ED (1993) Directional instability ofkinetocho re motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol 122: 859–875.
Snyder JA, McIntosh JR (1975) Initiation and growth of microtubules from mitotic centers in lysed mammalian cells. J Cell Biol 67: 744–760.
Steuer ER, Wordeman L, Schroer TA, Sheetz MP (1990) Localization ofcytoplasm ic dynein to mitotic spindles and kinetochores. Nature 345: 266–268.
Szollosi D, Calarco P, Donahue RP (1972) Absence of centrioles in the first and second meiotic spindles ofmouse oocytes. J Cell Sci 11: 521–541.
Tan EM, Rodnan GP, Garcia I, Moroi Y, Fritzler MJ, Peebles C (1980) Diversity ofantinucle ar antibodies in progressive systemic sclerosis. Anti-centromere antibody and its relationship to CREST syndrome. Arthritis Rheum 23: 617–625.
Telzer BR, Moses MJ, Rosenbaum JL (1975) Assembly of microtubules onto kinetochores ofisolated mitotic chromosomes ofHeLa cells. Proc Natl Acad Sci USA 72: 4023–4027.
Wilson EB (1925) The Cell in Development and Heredity, 3rd edn. New York: Macmillan.
Wise D, Cassimeris L, Rieder CL, Wadsworth P, Salmon ED (1991) Chromosome fiber dynamics and congression oscillations in metaphase PtK2 cells at 23 degrees C. Cell Motil Cytoskeleton 18: 131–142.
Witt PL, Ris H, Borisy GG (1980) Origin ofkinetoch ore microtubules in Chinese hamster ovary cells. Chromosoma 81: 483–505.
Wood KW, Sakowicz R, Goldstein LS, Cleveland DW (1997) CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91: 357–366.
Yajima J, Edamatsu M, Watai-Nishii J, Tokai-Nishizumi N, Yamamoto T, Toyoshima YY (2003). The human chromokinesin Kid is a plus end-directed microtubule-based motor. EMBO J 22: 1067–1074.
Yen TJ, Compton DA, Wise D et al. (1991) CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J 10: 1245–1254.
Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW (1992) CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature 359: 536–539.
Zhang D, Nicklas RB (1995) Chromosomes initiate spindle assembly upon experimental dissolution ofthe nuclear envelope in grasshopper spermatocytes. J Cell Biol 131: 1125–1131.
Zinkowski RP, Meyne J, Brinkley BR (1991). The centromere-kinetochore complex: a repeat subunit model. J Cell Biol 113: 1091–1110.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maiato, H., Sunkel, C.E. Kinetochore–microtubule interactions during cell division. Chromosome Res 12, 585–597 (2004). https://doi.org/10.1023/B:CHRO.0000036587.26566.81
Issue Date:
DOI: https://doi.org/10.1023/B:CHRO.0000036587.26566.81