Abstract
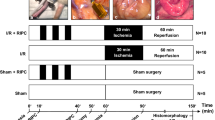
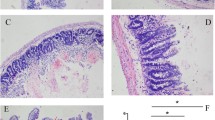
Ischemic preconditioning has shown to reduce apoptosis in the intestinal mucosa during ischemia/reperfusion. This study evaluated if the decrease of apoptotic events found during preconditioning could be related with a reduction of the substrate (i.e., xanthine/hypoxanthine) available for xanthine oxidase (XO). Animals were randomly assigned to the following study groups: C, control; I/R, ischemia/reperfusion; P+I/R, ischemic preconditioning; P+I/R+H/X, ischemic preconditioning plus hypoxanthine/xanthine, and P+I/R+H/X+Allo, ischemic preconditioning plus hypoxanthine/xanthine plus allopurinol. Caspase-3 activity, DNA fragmentation and TUNEL staining increased in the I/R group compared to control. Ischemic preconditioning (P+I/R group) was able to reverse these apoptotic variables to a level similar to that of control rats. The addition of hypoxanthine/xanthine to rats subjected to ischemic preconditioning (P+I/R+H/X group) showed the highest apoptotic activity; however, further addition of allopurinol (P+I/R+H/X+Allo group) decreased significantly apoptotic activity and events. In conclusion, intestinal ischemic preconditioning is able to reduce apoptosis during the following sustained ischemia/reperfusion event because of a reduced accumulation of xanthine/hypoxanthine nucleotide.
Similar content being viewed by others
References
Ishida T, Yakimuzu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock 1997; 8: 86–94.
Hotter G, Closa D, Prados M, et al. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun 1996; 222: 27–32.
McLaren AJ, Friend PJ. Trends in organ preservation. Transpl Int 2003; 16(10): 701–708.
Kosieradzki M. Mechanisms of ischemic preconditioning and its application in transplantation. Ann Transplant 2002; 7(3): 12–20.
Ferencz A, Szanto Z, Borsiczky B, et al. The effects of preconditioning on the oxidative stress in small-bowel autotransplantation. Surgery 2002; 132(5): 877–884.
Farber A, Connors JP, Friedlander RM, Wagner RJ, Powell RJ, Cronenwett JL. A specific inhibitor of apoptosis decreases tissue injury after intestinal ischemia-reperfusion in mice. J Vasc Surg 1999; 30(4): 752–760.
Fukuyama K, Iwakiri R, Noda T, et al. Apoptosis induced by ischemia-reperfusion and fasting in gastric mucosa compared to small intestinal mucosa in rats. Dig Dis Sci 2001; 46(3): 545–549.
Genescà M, Sola A, Miquel R, et al. Role of changes in tissular nucleotides on the development of apoptosis during ischemia/reperfusion in rat small bowel. Am J Pathol 2002; 161: 1839–1847.
Hotchkiss RS, Schmieg RE, Jr., Swanson PE, et al. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med 2000; 28(9): 3207–3217.
Ikeda H, Suzuki Y, Suzuki M, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 1998; 42(4): 530–537.
Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol 1998; 274(2Pt1): G270–276.
Bedirli A, Soyuer I, Muhtaroglu S, Guler I. Role of granuocyte-macrophage colony-stimulating factor on apoptosis induced by ischemia-reperfusion in the intestinal epithelium. Eur Surg Res 2003; 35: 357–362.
Liu H, McPherson BC, Yao Z. Preconditioning attenuates apoptosis and necrosis: Role of protein kinase C epsilon and-delta isoforms. Am J Physiol Heart Circ Physiol 2001; 281(1): H404–H410.
Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res 2002; 55(3): 438–455.
Raymond MA, Vigneault N, Luyckx V, Hebert MJ. Paracrine repercussions of preconditioning on angiogenesis and apoptosis of endothelial cells. Biochem Biophys Res Commun 2002; 291(2): 261–269.
Sindram D, Rudiger HA, Upadhya AG, Strasberg SM, Clavien PA. Ischemic preconditioning protects against cold ischemic injury through and oxidative stress dependent mechanism. J Hepatol 2002; 36(1): 78–84.
Cavalieri B, Perrelli MG, Aragno M, et al. Ischemic preconditioning attenuates the oxidant-dependent mechanisms of reperfusion cell damage and death in rat liver. Liver Transpl 2002; 8(11): 990–999.
Chien CT, Hsu SM, Chen CF, Lee PH, Lai MK. Hypoxic preconditioning reduces ischemia/referfusion-induced apoptosis cell death in rat kidney. Transplant Proc 2000; 32(7): 1653–1654.
Ravati A, Ahlemeyer B, Becker A, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species. Brain Res 2000: 23–32.
Zhang C, Rosenbaum DM, Shaikh AR, et al. Ischemic preconditioning attenuates apoptotic cell death in the rat retina. Invest Ophthalmol Vis Sci 2002; 43(9): 3059–3066.
Cinel I, Avalan D, Cinel L, et al. Ischemic preconditioning reduces intestinal epithelial apoptosis in rats. Shock 2003; 19(6): 588–592.
Fox IH. Metabolic basis for disorders of purine nucleotide degradation. Metabolism 1981; 30: 616–634.
Saugstad OD. Hypoxanthrine as an indicator of hypoxia: Its role in health and disease through free radical production. Pediatr Res 1988; 23: 143–150.
Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today 1994; 15: 1–4.
Cai J, Jones DP. Superoxide in apoptosis: Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 1998; 273: 11401–11404.
Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: Its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol 2000; 15: 109–120.
Sola A, Hotter G, Prats N, Xaus C, Gelpi E, Rosello-Catafau J. Modification of oxidative stress in response to intestinal preconditioning. Transplantation 2000; 69(5): 767–772.
Hotter G, Closa D, Prats N, Pi F, Gelpi E, Rosello-Catafau J. Free radical enhancement promotes leucocyte recruitment through a PAF and LTB4 dependent mechanism. Free Radic Biol Med 1997; 22(6): 947–954.
Hotter G, Closa D, Gelpi E, Prats N, Rosello-Catafau J. Role of xanthine oxidase and eicosanoids in development of pancreatic ischemia-reperfusion injury. Inflammation 1995; 19(4): 469–478.
Burton K. A study of the conditions and mechanism of diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 1956; 62: 315–323.
Jha HC, von Recklinghausen G, Zilliken F. Inhibition of in vitro microsomal lipid peroxidation by isoflavonoids. Biochem Pharmacol 1985; 34(9): 1367–1369.
Haglund U. Gut ischemia. Gut 1994; 35(Suppl 1): 73–76.
Shah KA, Shurey S, Green CJ. Apoptosis after intestinal ischemia-reperfusion injury: A morphological study. Transplantation 1997; 64(10): 1393–1397.
Wells CL. Colonization and translocation of intestinal bacterial flora. Transplant Proc 1996; 28(5): 2653–2656.
Parks D, Granger D. Xanthine oxidase: Biochemistry, distribution and physiology. Acta Physiol Scand 1986; 548: 87–99.
Hockenbery DM, Oltvai ZN, Yin XM, Milliman C, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993; 75: 241–251.
Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 2002; 9: 195–217.
Ciz M, Cizova H, Lojek A, Kubala L, Papezikova I. Ischemia/reperfusion injury of rat small intestine: The effect of allopurinol dosage. Transplant Proc 2001; 33: 2871–2873.
Albuquerque RG, Sanson AJ, Malangoni AM. Allopurinol protects enterocytes from hypoxia-induced apoptosis in vivo. J Trauma 2002; 53: 415–421.
Parra E, Gota R, Gamen A, Moros M, Azuara M, Granulomatous interstitial nephritis secondary to allopurinol treatment. Clin Nephrol 1995; 43(5): 350.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sola, A., Alfaro, V. & Hotter, G. Intestinal ischemic preconditioning: Less xanthine accumulation relates with less apoptosis. Apoptosis 9, 353–361 (2004). https://doi.org/10.1023/B:APPT.0000025812.45382.4d
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000025812.45382.4d