Abstract
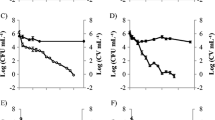
In Salmonella typhimurium, cadA has a role in virulence expression and is an inducible gene that responds to external lysine concentration. In this study, a strain of S. typhimurium carrying a cadA: lacZ fusion was used to determine if the induction of cadA occurred under different lysine concentrations and mildly acid conditions in the presence of short chain fatty acids. Aliquots of an 18-h culture of S. typhimurium were placed on fresh media containing different lysine concentrations at pH 5.8 adjusted by addition of HCl or by 1 M short chain fatty acids (SCFA, acetic, propionic and butyric acid) stock solution. After an induction period of 2 h, β-galactosidase activities were assayed. Expression of cadA in rich medium was significantly higher than that of minimal medium at neutral pH and different lysine concentrations. In contrast, at pH 5.8, there was a significant increase in cadA expression, particularly when pH was adjusted using HCl at all lysine levels. Addition of a mixture of organic acids yielded an overall lower cadA expression at all lysine levels studied when compared to HCl. However, each SCFA challenge (individual or as a mixture) caused a high level of expression, both at neutral and acidic pH. Based on these results it is apparent that in the presence of external lysine, SCFA and nutrient availability can influence cadA expression in S. typhimurium.
Similar content being viewed by others
References
Auger E.A., Redding K.E., Plumb T., Childs C., Meng S.-Y. and Bennett G.N. 1989. Construction of lac fusions to the inducible arginine-and lysine decarboxylase genes of Escherichia coli K12. Mol. Microbiol. 3: 609-620.
Bajaj V., Hwang C. and Lee C.A. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18: 715-727.
Bajaj V., Lucas R.L., Hwang C. and Lee C.A. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22: 703-714.
Baker D.H. and Han Y. 1994. Ideal amino acid profile for chicks during the first 3 weeks posthatching. Poultry Sci. 73: 1441-1447.
Bearson S., Bearson B. and Foster J.W. 1997. Acid responses in enterobacteria. FEMS Microbiol. Letts. 147: 173-180.
Boctor A.M. and Harper A.E. 1968. Measurement of available lysine in heated and unheated foodstuffs by chemical and biological methods. J. Nutr. 94: 289-296.
Carpenter K.J. and Booth V.H. 1973. Damage to lysine in food processing: its measurement and its significance. Nutr. Abstr. Rev. 43: 423-451.
Cherrington C.A., Hinton M., Mead G.C. and Chopra I. 1991. Organic acids: chemistry, antibacterial activity and practical applications. Adv. Microbiol. Physiol. 32: 87-108.
Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P.E. and MacFarlane G.T. 1987. Short-chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221-1227.
Durant J.A., Corrier D.E. and Ricke S.C. 2000a. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. J. Food Prot. 63: 573-578.
Durant J.A., Lowry V.K., Nisbet D.J., Stanker L.H., Corrier D.E. and Ricke S.C. 2000b. Short chain fatty acids alter HEp-2 cell association and invasion by stationary growth phase Salmonella typhimurium. J. Food Sci. 65: 1206-1209.
Durant J.A., Lowry V.K., Nisbet D.J., Stanker L.H., Corrier D.E. and Ricke S.C. 2000c. Late logarithmic Salmonella typhimurium HEp-2 cell association and invasion response to short-chain fatty acid addition. J. Food Safety 20: 1-11.
Erickson A.M., Zabala Díaz I.B., Kwon Y.M. and Ricke S.C. 2000. A bioluminescent Escherichia coli auxotroph for use in an in vitro lysine availability assay. J. Microbiol. Meth. 40: 207-212.
Finlay B.B. and Falkow S. 1989. Salmonella as an intracellular parasite. Mol. Microbiol. 3: 1833-1841.
Foster J.W. and Spector M.P. 1995. How Salmonella survive against the odds. Ann. Rev. Microbiol. 49: 145-174.
Hardin M.D., Acuff G.R., Lucia L.M., Oman J.S. and Savell J.W. 1995. Comparison of methods for contamination removal from beef carcass surfaces. J. Food Prot. 58: 368-374.
Hinton M. and Linton A.H. 1988. Control of salmonella infections in broiler chickens by the acid treatment of their feed. Vet. Rec. 123: 416-421.
Jay J.M. 1997. Modern Food Microbiology. 5th edn. Chapman and Hall, New York, NY.
Kwon Y.M. and Ricke S.C. 1998a. Induction of acid resistance of Salmonella typhimurium by exposure to short-chain fatty acids. Appl. Environ. Microbiol. 64: 3458-3463.
Kwon Y.M. and Ricke S.C. 1998b. Survival of a Salmonella typhimurium poultry isolate in the presence of propionic acid under aerobic and anaerobic conditions. Anaerobe 4: 251-256.
Kwon Y.M., Park S.Y., Birkhold S.G. and Ricke S.C. 2000. Induction of resistance of Salmonella typhimurium to environmental stresses by exposure to short-chain fatty acids. J. Food Sci. 65: 1037-1040.
MacFarlane G.T., Gibson G.R. and Cummings J.H. 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72: 57-64.
Mason T.G. and Richardson G. 1981. A review Escherichia coli and the human gut: some ecological considerations. J. Appl. Bacteriol. 51: 1-16.
Mekalanos J.J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174: 1-7.
Meng S.-Y. and Bennett G.N. 1992a. Regulation of the Escherichia coli cad operon: location of a site required for acid induction. J. Bacteriol. 174: 2670-2678.
Meng S.-Y. and Bennett G.N. 1992b. Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J. Bacteriol. 174: 2659-2669.
Miller J.H. 1972. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Neely M.N. and Olson E.R. 1996. Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J. Bacteriol. 178: 5522-5528.
Nordheim J.P. and Coon C.N. 1984. A comparison of four methods for determining available lysine in animal protein levels. Poultry Sci. 63: 1040-1051.
Park Y.-K., Bearson B., Bang S.H., Bang I.S. and Foster J.W. 1996. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol. Microbiol 20: 605-611.
Sabo D.L., Bucker E.A., Byers B., Waron H. and Fisher E.H. 1974. Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry 13: 662-670.
Salanitro J.P., Blake I.G., Muirhead P.A., Maglio M. and Goodman J.R. 1978. Bacteria isolated from the duodenum, ileum, and cecum of young chicks. Appl. Environ. Microbiol. 35: 782-790.
Sambrook J., Frisch E.F. and Maniatis T. 1989. Molecular Cloning-A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press, Plainview, NY.
Scott Merrell D. and Camilli A. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34: 836-849.
Srivastava R., Kumar D. and Srivastava B.S. 1997. Recombinant Mycobacterium aurum expressing Escherichia coli ?-galactosidase in high throughput screening of antituberculosis drugs. Biochem. Biophys. Res. Comm. 240: 536-539.
Tabor H., Hafner E.W. and Tabor C.W. 1980. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J. Bacteriol. 144: 952-956.
Watson N., Dunyak D.S., Rosey E.L., Slonczewski J.L. and Olson E.R. 1992. Identification of elements involved in transcription regulation of the Escherichia coli cad operon by external pH. J. Bacteriol. 174: 530-540.
Wolin M.J. 1981. Fermentation in the rumen and human large intestine. Science 213: 1463-1468.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zabala Díaz, I.B., Ricke, S.C. Influence of short chain fatty acids and lysine on Salmonella typhimurium cadA expression. Antonie Van Leeuwenhoek 85, 45–51 (2004). https://doi.org/10.1023/B:ANTO.0000020271.11533.71
Issue Date:
DOI: https://doi.org/10.1023/B:ANTO.0000020271.11533.71