Abstract
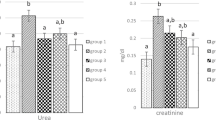
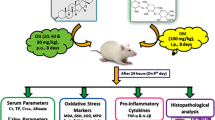
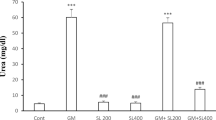
Gentamicin (GM) is an antibiotic whose clinical use is limited by its nephrotoxicity. Experimental evidences suggest a role of reactive oxygen species in GM-induced nephrotoxicity. In this work we explored the effect of diallyl sulfide (DAS), a garlic-derived compound with antioxidant properties, on GM-induced nephrotoxicity. Four groups of rats were studied: (1) Control, treated intragastrically with olive oil as a vehicle, (2) GM, treated subcutaneously with GM (125 mg/kg/day for 4 days), (3) DAS, treated intragastrically with DAS (50 mg/kg/day for 4 days), and (4) GM + DAS. Nephrotoxicity was made evident by: (1) the increase in creatinine and blood urea nitrogen in serum, (2) the increase in urinary excretion of N-acetyl-β-D-glucosaminidase and total protein, and (3) necrosis of proximal tubular cells. These functional and structural alterations were prevented or ameliorated by DAS treatment. In addition, GM increased levels of renal oxidative stress markers nitrotyrosine and protein carbonyl groups which were also ameliorated by DAS in GM + DAS group. The mechanism by which DAS has a protective effect on GM-induced nephrotoxicity may be related, at least in part, to the decrease in oxidative stress in renal cortex.
Similar content being viewed by others
References
Ali BH: Gentamicin nephrotoxicity in humans and animals: Some recent research. Gen Pharmacol 26: 1477-1487, 1995
Lerner SA, Schmitt BA, Seligosohn R, Matz GJ: Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med 80: 98-104, 1986
Laurent G, Tulkens PM: Aminoglycoside nephrotoxicity: Cellular and molecular aspects. ISI Atlas of Science: Pharmacology 1: 40-44, 1987
Silverblatt FJ, Kuehn C: Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int 15: 335-345, 1975
Ueda N, Mayeux PR, Baliga R, Shah SV: Oxidant mechanisms in acute renal failure. In: B.A. Molitoris, W.F. Finn (eds). Acute Renal Failure. A Companion to Brenner and Rector's The Kidney. W.B. Saunders Company Press, Philadelphia, 2001, pp 60-77
Martinez-Salgado C, Eleno N, Tavares P, Rodriguez-Barbero A, Garcia-Criado J, Bolanos JP, Lopez-Novoa JM: Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int 62: 1682-1692, 2002
Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Britti D, De Sarro A, Pierpaoli S, Caputi AP, Masini E, Salvemini D: A role for superoxide in gentamicin-mediated nephropaty in rats. Eur J Pharmacol 450: 67-76, 2002
Morales AI, Buitrago JM, Santiago JM, Fernandez-Tagarro M, López-Novoa JM, Perez-Barriocanal F: Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal 4: 893-898, 2002
Pedraza-Chaverrí J, Maldonado PD, Medina-Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, Hernández-Pando R, Ibarra-Rubio ME: Garlic ameliorates gentamicin nephrotoxicity: Relation to antioxidant enzymes. Free Radic Biol Med 29: 602-611, 2000
Yin MC, Hwang SW, Chan KC: Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J Agric Food Chem 50: 6143-6147, 2002
Grudzinski IP, Frankiewicz-Jozko A, Bany J: Diallyl sulfide — a flavour component from garlic (Allium sativum) attenuates lipid peroxidation in mice infected with Trichinella spiralis. Phytomedicine 8: 174-177, 2001
Lawson LD: The composition and chemistry of garlic cloves and processed garlic. In: H.P. Koch, L.D. Lawson (eds), Garlic. The Science and Therapeutic Application of Allium sativum L. and Related Species. Williams & Wilkins, Baltimore, 1996, pp 37-107
Rosen RT, Hiserodt RD, Fukuda EK, Ruiz RJ, Zhou Z, Lech J, Rosen SL, Hartman TG: Determination of allicin, S-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J Nutr 131: 968S-971S, 2001
Taucher J, Hansel A, Jordan A, Lindinger W: Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J Agric Food Chem 44: 3778-3782, 1996
Pentz R, Siegers CP: Garlic preparations: methods for qualitative and quantitative assessment of their ingredients. In: H.P. Koch, L.D. Lawson (eds). Garlic. The Science and Therapeutic Application of Allium sativum L. and Related Species. Williams & Wilkins, Baltimore, 1996, pp 109-134
Pedraza-Chaverrí J, Granados-Silvestre MA, Medina-Campos ON, Hernández-Pando R: Effect of the in vivo catalase inhibition on aminonucleoside nephrosis. Free Radic Biol Med 27: 245-253, 1999
Barrera D, Maldonado PD, Mcdina-Campos ON, Hernández-Pando R, Ibarra-Rubio ME, Pedraza-Chaverrí J: HO-1 induction attenuates renal damage and oxidative stress induced by K2Cr2O7. Free Radic Biol Med 34: 1390-1398, 2003
Sener G, Sehirli AO, Altunbas HZ, Ersoy Y, Paskaloglu K, Arbak S, Ayanoglu-Dulger G: Melatonin protects against gentamicin-induced nephrotoxicty in rats. J Pineal Res 32: 231-236, 2002
Mazzon E, Britti D, De Sarro A, Caputi AP, Cuzzocrea S: Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur J Pharmacol 424: 75-83, 2001
Ali BH: The effect of treatment with the medicinal plant Rhazya stricta decne on gentamicin nephrotoxicity in rats. Phytomedicine 9: 385-389, 2002
Sai-Kato K, Umemura T, Takagi A, Hasegawa R, Tanimura A, Kurokawa Y: Pentachlorophenol-induced oxidative DNA damage in mouse liver and protective effect of antioxidants. Food Chem Toxicol 33: 877-882, 1995
Sahu SC, Washington MC: Iron-mediated oxidative DNA damage detected by fluorometric analysis of DNA unwinding in isolated rat liver nuclei. Biomed Environ Sci 4: 232-241, 1991
Fanelli SL, Castro GD, de Toranzo EG, Castro JA: Mechanisms of the preventive properties of some garlic components in the carbon tetrachloride-promoted oxidative stress. Diallyl sulfide; diallyl disulfide; allyl mercaptan and allyl methyl sulfide. Res Commun Mol Pathol Pharmacol 102: 163-174, 1998
Cai XJ, Block E, Uden PC, Quimby BD, Sullivan JJ: Allium chemistry: Identification of natural abundance organoselenium compounds in human breath after ingestion of garlic using gas chromatography with atomic emission detection. J Agric Food Chem 43: 1751-1753, 1995
Laakso I, Seppanen-Laakso T, Hiltunen R, Muller B, Jansen H, Knobloch K: Volatile garlic odor components: Gas phases and adsorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Med 55: 257-261, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pedraza-Chaverrí, J., Maldonado, P.D., Barrera, D. et al. Protective effect of diallyl sulfide on oxidative stress and nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem 254, 125–130 (2003). https://doi.org/10.1023/A:1027372102135
Issue Date:
DOI: https://doi.org/10.1023/A:1027372102135