Abstract
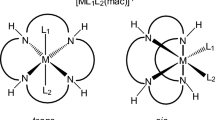
Aquation of [Cr(pic)3]0 and [Cr(pic)2(OH)]2 0 in aqueous HClO4 solutions leads to formation of the common product – [Cr(pic)2(H2O)2]+. The first, reversible stage, the ring opening via Cr—N bond breaking in [Cr(pic)3]0 is followed by the second, rate-determining step – one-end bonded pic ligand liberation. In the case of the [Cr(pic)2(OH)]2 0 complex, the first faster stage produces the singly bridged dimer, which undergoes cleavage into the parent monomers in the second, much slower step. The subsequent aquation of [Cr(pic)2(H2O)2]+ is extremely slow and leads to [Cr(pic)(H2O)4]2+ formation, which practically does not undergo further ligand substitution under the conditions applied. Kinetics of the first aquation stage for [Cr(pic)3]0 and of the second step for [Cr(pic)2(OH)]2 0 were studied spectrophotometrically in the 0.1–1.0 M HClO4 range at I = 1.0 M. The observed pseudo-first order rate constant for [Cr(pic)3]0 decreases with [H+] increase according to the rate law: k obs = k 1 + k −1 Q 1/[H+], where k 1 and k −1 are the rate constants of the forward and the reverse processes in the unprotonated substrate and Q 1 is the protonation constant of the pyridine nitrogen atom. In the case of the [Cr(pic)2(OH)]2 0 complex, the rate for the singly bridged dimer cleavage does not depend on [H+]. The activation parameters for the chelate-ring opening in [Cr(pic)3]0 and for the singly bridged dimer cleavage have been determined and discussed. Some kinetic data of the slow, second aquation stage for the [Cr(pic)3]0 complex and of the fast, first aquation stage for the doubly bridged dimer have been studied; for both reactions the rate increases linearly with the increase in [H+].
Similar content being viewed by others
References
Committee on Animal Nutrition, Board of Agriculture, National Research Council, The Role of Chromium in Animal Nutrition, National Academy Press, Washington, DC, 1997.
K.F. Kingry, A.C. Royer and J.B. Vincent, J. Inorg. Biochem., 72, 79 (1998).
D.M. Stearns, J.P. Wise Sr., S.R. Patierno and K.E. Wetterhahn, FASEB J., 9, 1643 (1995).
J.K. Speetjens, R.A. Collins, J.B. Vincent and S.A. Woski, Chem. Res. Toxicol., 12, 483 (1999).
D.M. Stearns, S.M. Silveria, K.K. Wolf and A.M. Luke, Mutational Res., 513, 135 (2002).
D.M. Stearns and W.H. Armstrong, Inorg. Chem., 31, 5178 (1992).
N.E. Chakov, R.A. Collins and J.B. Vincent, Polyhedron, 18, 2891 (1999).
E. Kita, P. Kita and G. Uścińska, Polish J. Chem., 72, 1949 (1998).
C. Cativiela, J.-L. Dejardin, J. Elguero, J.I. Garcia, E. Gonzales and J.A. Mayoral, Collect. Czech. Chem. Commun., 55, 72 (1990).
E. Kita and M. Łaczna, Trans. Met. Chem., 26, 510 (2001).
J. Springborg and H. Toftlund, Acta Chem. Scand., A 30, 171 (1976).
L. Spiccia and W. Marty, Polyhedron, 10, 619 (1991).
L. Spiccia, Polyhedron, 10, 1865 (1991).
M.R. Grace and L. Spiccia, Polyhedron, 10, 2389 (1991).
S.J. Crimp and L. Spiccia, J. Chem. Soc. Dalton Trans., 1051, (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kita, E., Szabłowicz, M. Kinetics and mechanism of the first aquation stage for the [Cr(pic)3]0 and [Cr(pic)2(OH)]2 0 complexes in HClO4 solutions. Transition Metal Chemistry 28, 698–706 (2003). https://doi.org/10.1023/A:1025469431212
Issue Date:
DOI: https://doi.org/10.1023/A:1025469431212