Abstract
Purpose. To investigate the relationship between mean dissolution time (MDT) and dose/solubility ratio (q) using the diffusion layer model.
Methods. Using the classic Noyes-Whitney equation and considering a finite dose, we derived an expression for MDT as a function of q under various conditions. q was expressed as a dimensionless quantity by taking into account the volume of the dissolution medium. Our results were applied to in vitro and in vivo data taken from literature.
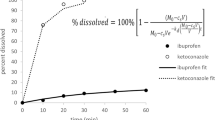
Results. We found that MDT depends on q when q > 1 and is infinite when q > 1 and that the classic expression of MDT = 1/k, where k is the dissolution rate constant, holds only in the special case of q = 1. For the case of perfect sink conditions, MDT was found to be proportional to dose. Using dissolution data from literature with q < 1, we found better estimates of MDT when dependency on dose/solubility ratio was considered than with the classic approach. Prediction of dissolution limited absorption was achieved for some of the in vivo drug examples examined.
Conclusion. The mean dissolution time of a drug depends on dose/solubility ratio, even when the model considered is the simplest possible. This fact plays an important role in drug absorption when absorption is dissolution limited.
Similar content being viewed by others
REFERENCES
K. Yamaoka, T. Nakagawa, and T. Uno. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 6:547-558 (1978).
D. Brockmeier. Mean time concept and component analysis in pharmacokinetics. Int. J. Clin. Pharmacol. Ther. 37:555-561 (1999).
D. Brockmeier, D. Voegele, and H.M von Hattinberg. In vitro-in vivo correlation, a time sampling problem. Arzneim. Forsch. Drug Res. 33:598-601 (1983).
P. Lansky and M. Weiss. Does the dose-solubility ratio affect the mean dissolution time of drugs? Pharm. Res. 16:1470-1476 (1999).
A. S. Noyes and W. R. Whitney. The rate of solution of solid substances in their own solutions. J. Amer. Chem. Soc. 19:930-934 (1897).
Y. Tanagawara, K. Yamaoka, T. Nakagawa, and T. Uno. New method for the evaluation of in vitro dissolution time and disintegration time. Chem. Pharm. Bul. 30:1088-1090 (1982).
R. K. Brazzell and S. A. Kaplan. Factors affecting the accuracy of estimated mean absorption times and mean dissolution times. J. Pharm. Sci. 72:713-715 (1983).
K. C. Khoo, M. Gibaldi, and R. K. Brazzell. Comparison of statistical moment parameters to Cmax and tmax for detecting differences in in vivo dissolution rates. J. Pharm. Sci. 74:1340-1342 (1985).
N. D. Eddington, P. Marroum, R. Uppoor, A. Hussain, and L. Augsburger. Development and internal validation of an in vitro-in vivo correlation for a hydrophilic metoprolol tartrate extended release tablet formulation. Pharm. Res. 15: 466-473 (1998)
N. D. Eddington, P. Marroum, R. Uppoor, A. Hussain, and L. Augsburger. Erratum. Pharm. Res. 15:1320(1998).
L. X. Yu and J. R. Crison. and G.L Amidon. Compartmental transit and dispersion model analysis for small intestinal transit flow in humans. Int. J. Pharm. 140:111-118 (1996).
L. X. Yu. An integrated model for determining causes of poor oral drug absorption. Pharm. Res. 16:1883-1887 (1999).
J. B. Dressman and D. Fleisher. Mixing-tank model for predicting dissolution rate control of oral absorption. J. Pharm. Sci. 75:109-116 (1986).
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413-420 (1995).
L. X. Yu, G. L. Amidon, J. E. Polli, H. Zhao, M. U. Mehta, D. P. Conner, V. P. Shah, L. J. Lesko, M. L. Chen, V. H. Lee, and A. S. Hussain. Biopharmaceutics classification system: the scientific basis for biowaiver extensions. Pharm. Res. 19:921-925 (2002).
L. X. Yu, E. Lipka, and G. L. Amidon. Transport approaches to the biopharmaceutical design of oral drug delivery systems. Adv. Drug Deliv. Rev. 19:359-376 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rinaki, E., Dokoumetzidis, A. & Macheras, P. The Mean Dissolution Time Depends on the Dose/Solubility Ratio. Pharm Res 20, 406–408 (2003). https://doi.org/10.1023/A:1022652004114
Issue Date:
DOI: https://doi.org/10.1023/A:1022652004114