Abstract
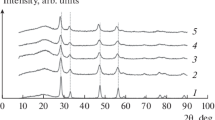
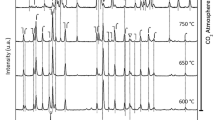
La1.867Th0.100CuO4 was prepared by means of the citric acid complexing method. The reduction–oxidation (redox) properties of this composite oxide have been investigated by using the XRD, TGA, EPR, TPD, and SEM methods. The fresh (non-reduced) La1.867Th0.100CuO4 catalyst is single phase with tetragonal K2NiF4-type structure. There were three reduction steps observed over La1.867Th0.100CuO4 in the temperature ranges of 25–100, 100–300, and 300–500 °C, respectively. After reduction at 300 °C, the material still retained its original single phase but there were oxygen vacancies generated in the lattice. After reduction at 500 °C, it decomposed to a mixture of oxides. In the course of reduction, trapped electrons were generated. During the oxidation of the reduced sample, O −2 was detected. Apparently, oxygen vacancies are able to stabilise O −2 on the surface of the -1ptcatalyst. NO adsorption on both the fresh and reduced La1.867Th0.100CuO4 samples generated NO radicals and O −2 species. On a La1.867Th0.100CuO4 sample reduced at 300 °C, [O2NO2]2− was generated in NO adsorption and decomposed to N2 and O2− at ca. 730 °C. After reduction, the O −2 inside the La1.867Th0.100CuO4 lattice became more mobile and participated in the decomposition of [O2NO2]2−. The fresh (non-reduced) La1.867Th0.100CuO4 sample with cation defects in its lattice shows higher NO decomposition activity than the fresh La2CuO4 sample in which there are no cation defects. The 300 °C-reduced La1.867Th0.100CuO4 with cation defects and oxygen vacancies is more active than the fresh one for NO decomposition. The redox action between Cu+ and Cu2+ is an essential process for NO decomposition.
Similar content being viewed by others
References
K. Tabata and M. Misono, Catal. Today 8 (1990) 249.
Z.L. Yu, L.Z. Gao, S.Y. Yuan and Y. Wu, J. Chem. Soc. Faraday Trans. 88 (1992) 3245.
S.D. Peter, E. Garbowski, N. Guilhaume, V. Perrichon and M. Primet, Catal. Lett. 54 (1998) 79.
L.Z. Gao, Z.L. Yu and Y. Wu, in: Proc. 34th IUPAC Congr., 1993, p. 730.
L.Z. Gao, Z.L. Yu and Y. Wu, Acta Chim. Sinica 55 (1997) 56.
M. Anpo, M. Matsuoka, Y. Shioya, H. Yamashita, E. Giamello, C. Morterra, M. Che, H.H. Paterson, S. Webber, S. Oullete and M.A. Fox, J. Phys. Chem. 98 (1994) 5744.
E. Giamello, D. Murphy, G. Magacca, C. Morterra, Y. Shioya, T. Nomura and M. Anpo, J. Catal. 136 (1992) 510.
J.W. London and A.T. Bell, J. Catal. 31 (1973) 96.
N. Mizuno, M. Yamato and M. Tanaka, Chem. Mater. 1 (1989) 232.
V.I. Pârvulescu, P. Grange and B. Delmon, Catal. Today 46 (1998) 233.
G.J. Millar, A. Canning, G. Rose, B. Wood, L. Trewartha and I.D.R. Mackinnon, J. Catal. 183 (1999) 169.
E.S.J. Lox and B.H. Engler, Handbook of Heterogeneous Catalysis, Vol. 4, eds. G. Ertl, H. Knözinger and J. Weitkamp (1997) p. 1628.
L.K. Gushee and R. Ward, J. Am. Chem. Soc. 79 (1957) 5601.
D.C. Harris and T.A. Hewton, J. Solid State Chem. 69 (1987) 182.
C.T. Au and X.P. Zhou, J. Chem. Soc. Faraday Trans. 92 (1996) 1793.
C. Morterra, E. Giamello, G. Gerrato, G. Centi and S. Perathoner, J. Catal. 179 (1989) 111.
A. Martínez-Arias, J. Sorria, J.C. Conesa, X.L. Seoane, A. Arcoya, and R. Cataluña, J. Chem. Soc. Faraday Trans. 91 (1995) 1679.
C. Oliva, L. Forni, A.M. Ezerets, I.E. Mukovozov and A.V. Vishniakov, J. Chem. Soc. Faraday Trans. 94 (1998) 587.
Z.X. Zhang and K.J. Klabunde, Inorg. Chem. 31 (1992) 1706.
Z. Zhao, X.G. Yang and Y. Wu, Sci. China B 28 (1998) 31.
M. Iwamoto, H. Furukawa and S. Kagawa, in: New Developments in Zeolite Science and Technology, Stud. Surf. Sci. Catal., Vol. 28, eds. Y. Murakami, A. Iijima and J.W. Ward (Elsevier, Amsterdam, 1986) p. 943.
Z. Sojka, M. Che and E. Giamello, J. Phys. Chem. B 101 (1997) 4831.
H. Yasuda, T. Nitadori, N. Mizuno and M. Misono, Bull. Chem. Soc. Jpn. 66 (1993) 3492.
F. Munakata, Y. Akimune, Y. Shichi, M. Akutsu, H. Yamaguchi and Y. Inoue, J. Chem. Soc. Chem. Commun. (1997) 63.
J.Y. Lin, A.T.S. Wee, K.L. Tan, K.G. Neoh and W.K. Teo, Inorg. Chem. 32 (1993) 5322.
H.X. Dai, C.F. Ng and C.T. Au, Catal. Lett. 57 (1999) 115.
A. Gervasini, P. Carniti and V. Ragaini, Appl. Catal. B 22 (1999) 201.
M. Iwamoto, H. Yahiro, K. Tanda, N. Mizuno, Y. Mine and S. Kagawa, J. Phys. Chem. 95 (1991) 3727.
Z. Chajar, M. Primet and H. Praliaud, J. Catal. 180 (1998) 279.
M. Shelf, Chem. Rev. 95 (1995) 209.
Y.F. Chang and J.G. McCarty, J. Catal. 178 (1998) 408.
J. Valyon and W.K. Hall, Catal. Lett. 19 (1993) 109.
J. Valyon and W.K. Hall, J. Catal. 143 (1993) 520.
J. Valyon, W.S. Millman and W.K. Hall, Catal. Lett. 24 (1994) 215.
J. Sarkany, J.L. D'Itri and W.M.H. Sachtler, Catal. Lett. 16 (1992) 241.
G.D. Lei, B.J. Adelman, J. Sarkany and W.M.H. Sachtler, Appl. Catal. B 5 (1995) 245.
S.C. Larsen, A.W. Aylor, A.T. Bell and J.A. Reimer, J. Phys. Chem. 98 (1994) 11533.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gao, L., Au, C. Studies on the redox behaviour of La1.867Th0.100CuO4 and its catalytic performance for NO decomposition. Catalysis Letters 65, 91–98 (2000). https://doi.org/10.1023/A:1019077507513
Issue Date:
DOI: https://doi.org/10.1023/A:1019077507513