Abstract
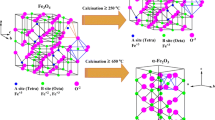
The thermal decomposition of Fe2(SO4)3 in air has been investigated using transmission Mössbauer spectroscopy, CEMS and X-ray powder diffraction. The hexagonal α-Fe2O3 and cubic β-Fe2O3 with spinel structure have been identified as products of the decomposition. The influence of the Fe2(SO4)3 particle size on the yield of β-Fe2O3 in the final product is pointed out and discussed. The size of particles and the calcination temperature are the most important kinetic factors determining the phase composition (β-Fe2O3/α-Fe2O3) of iron (III) oxide.
Similar content being viewed by others
References
L. Ben-Dor, E. Fischbein, I. Felner and Z. Kalman, J. Electrochem. Soc. 124 (1977) 451.
T. Muruyama and T. Kanagawa, J. Electrochem. Soc. 143 (1996) 1675.
T. Gonzales-Carreno, M.P. Morales and C.J. Serna, J. Mater. Sci. Lett. 13 (1994) 381.
Y. Ikeda, M. Takano and Y. Bando, Bull. Inst. Chem. Res. Kyoto Univ. 64 (1986) 249.
E.R. Bauminger, L. Ben-Dor, I. Felner, E. Fischbein, I. Nowik and S. Ofer, Physica B 86-88 (1977) 910.
L. Ben-Dor and E. Fischbein, Acta Cryst. B 32 (1976) 667.
D. Wiarda, T. Wenzel, M. Uhrmacher and K.P. Lieb, J. Phys. Chem. Solids 53 (1992) 1199.
D. Wiarda and G. Weyer, Internat. J. Mod. Phys. B 7 (1993) 353.
P.G. Coombs and Z.A. Munir, J. Therm. Anal. 35 (1989) 967.
H. Tagawa, Thermochim. Acta 80 (1984) 23.
A. Vertes and B. Zsoldos, Acta Chim. Acad. Sci. Hungar. 65 (1970) 261.
E.V. Margulis, M.M. Shokarev, L.A. Savtchenko, N.I. Kopylov and L.I. Bejsekeeva, Zh. Neorg. Khim. 16 (1971) 734.
A.H. Kamel, Z. Sawires, H. Khalifa, S.A. Saleh and A.M. Abdallah, J. Appl. Chem. Biotechnol. 22 (1972) 591.
H.M. Ismail, M.I. Zaki, A.M. Hussein and M.N. Magar, Powder Technol. 63 (1990) 87.
J. Milbauer, in: The Preparation of Inorganic Compounds (Czech Chemical Society, Praha, 1947) p. 148.
Powder Diffraction File 1997, International Center for Diffraction Data, Pennsylvania, USA.
H. Braun and K.J. Gallagher, Nature Phys. Sci. 240 (1972) 13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zboril, R., Mashlan, M., Krausova, D. et al. Cubic β-Fe2O3 as the product of the thermal decomposition of Fe2(SO4)3 . Hyperfine Interactions 120, 497–501 (1999). https://doi.org/10.1023/A:1017018111071
Issue Date:
DOI: https://doi.org/10.1023/A:1017018111071