Abstract
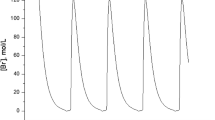
We investigate the steady-state multiplicity exhibited by the reaction of a fuel/air mixture in a continuously stirred tank reactor. The chemical mechanism used is a modification of a scheme due to Sal'nikov. We consider four cases; corresponding to the choice of fuel fraction, inflow temperature, inflow pressure, or precursor decay rate as the primary bifurcation parameter. From the perspective of fire-retardancy, the case when the fuel fraction is varied is the most important. In this case the steady-state diagrams provide a basis for a systematic investigation into the effectiveness of gas-phase active fire retardants.
Similar content being viewed by others
References
L.F. Razon and R.A. Schmitz, Multiplicities and instabilities in chemically reacting systems-a review, Chem. Engrg. Sci. 42(5) (1987) 1005–1047.
V. Caprio, A. Insola and R. Barbella, Preflame processes in the ozone activated oxidation of methlycyclopentane, in: Combustion Institute European Symposium 1973, ed. F.J. Weinberg (Academic Press, New York, 1973) pp. 99–104.
V. Caprio, A. Insola and P.G. Lignola, Isobutane cool flames investigation in a continuous stirred tank reactor, in: Sixteenth International Symposium on Combustion(The Combustion Institute, 1976) pp. 1155–1163.
P.G. Felton, B.F. Gray and N. Shank, Low temperature oxidation in a stirred flow reactor-II. Acetaldehyde, Combustion and Flame 27 (1976) 363–376.
B.F. Gray and P.G. Felton, Low-temperature oxidation in a stirred-flow reactor-I. Propane, Combustion and Flame 23 (1974) 295–304.
B.F. Gray, P.G. Felton and N. Shank, A theoretical and experimental study of the low temperature oxidation of acetaldehyde, in: 2nd European Symposium on Combustion(The Combustion Institute, 1975) p. 103.
T. Faravelli, P. Gaffuri, E. Ranzi and J.F. Griffiths, Detailed thermokinetic modelling of alkane autoignition as a tool for the optimization of performance of internal combustion engines, Fuel 77(3) (1998) 147–155.
J.F. Griffiths, Thermokinetic oscillations in homogeneous gas-phase oxidations, in: Oscillations and Travelling Waves in Chemical Systems, eds. R.J. Field and M. Burger (Wiley, New York, 1985) pp. 529–564.
P. Gray and S.K. Scott, Experimental systems 2: Gas-phase reactions, in: Chemical Oscillations and Instabilities: Nonlinear Chemical Kinetics, International Series of Monographs on Chemistry, Vol. 21 (Clarendon Press, 1990) chapter 15.
J.F. Griffiths and S.K. Scott, Thermokinetic interactions: Fundamentals of spontaneous ignition and cool flames, Progr. Energy Combust. Sci. 13 (1987) 161–197.
I.Y. Sal'nikov, A thermokinetic model of homogeneous periodic reactions, Doklady Akademii Nauk SSSR 60(3) (1948) 405–408 (in Russian).
I.Y. Salnikov, Contribution to the theory of the periodic homogenous chemical reactions II. A thermokinetic self-excited oscillating model, Zhurnal Fizicheskoi Khimii 23 (1949) 258–272 (in Russian).
L.P. Russo and B.W. Bequette, Impact of process design on the multiplicity behaviour of a jacketed exothermic CSTR, AIChe J. 41(1) (1995) 135–147.
C.T. Ewing, J.T. Hughes and H.W. Carhart, The extinction of hydrocarbon flames based on the heat-absorption processes which occur in them, Fire and Materials 8(3) (1984) 148–155.
B.F. Gray, Unified theory of explosions, cool flames and two-stage igntions, Part 1, Trans. Faraday Soc. 65 (1969) 1603–1613.
C.H. Yang and B.F. Gray, Unified theory of explosions, cool flames and two stage ignitions, part 2, Trans. Faraday Soc. 65 (1969) 1614–1622.
B.F. Gray and M.J. Roberts, Analysis of chemical kinetic systems over the entire parameter space I. The Sal'nikov thermokinetic oscillator, Proc. Roy. Soc. London Ser. A 416 (1988) 391–402.
B.F. Gray and M.J. Roberts, An asymptotic analysis of the Sal'nikov thermokinetic oscillator, Proc. Roy. Soc. London Ser. A 416 (1988) 425–441.
P. Gray, Instabilities and oscillations in chemical reactions in closed and open systems, Proc. Roy. Soc. London Ser. A 415 (1988) 1–34.
P. Gray, S.R. Kay and S.K. Scott, Oscillations of an exothermic reaction in a closed system I. Approximate (exponential) represenation of an Arrhenius temperature-dependence, Proc. Roy. Soc. London Ser. A 416 (1988) 321–341.
S.R. Kay and S.K. Scott, Oscillations of simple exothermic reactions in a closed system II. Exact Arrhenius kinetics, Proc. Roy. Soc. London Ser. A 416 (1988) 343–359.
J.G. Burnell, J.G. Graham-Eagle, B.F. Gray and G.C. Wake, Determination of critical ambient temperature for thermal ignition, IMA J. Appl. Math. 42 (1989) 147–154.
B.F. Gray, J.H. Merkin and G.C. Wake, Disjoint bifurcation diagrams in combustion systems, Math. Comput. Modelling 15(11) (1991) 25–33.
B.F. Gray and G.C. Wake, On the determination of critical ambient temperatures and critial initial temperatures for thermal ignition, Combustion and Flame 71 (1988) 101–104.
L.K. Forbes, Limit-cycle behaviour in a model chemical reaction: the Sal'nikov thermokinetic oscillator, Proc. Roy. Soc. London Ser. A 430 (1990) 641–651.
H.N. Moreira and W. Yuquan, Sufficient conditions for the Sal'nikov equation to have at least two limit cycles, J. Math. Chem. 15 (1994) 63–72.
L.K. Forbes and B.F. Gray, Forced oscillations in an exothermic chemical reaction, Dynamics and Stability of Systems 9(3) (1994) 253–269.
E.J. Delgado R., Complex dynamics in a simple exothermic reaction model, Latin American Applied Research 24 (1994) 109–115.
E.J. Delgado, Chaos in a spherical chemical reactor, Boletin Sociedad Chilena Quimica 40 (1995) 17–24.
E.J. Delgado, A.F. Münster and F.W. Schneider, Chaos control and tracking of periodic states in a forced thermokinetic oscillator model, Berichte der Bunsen-Gesellschaft Physical Chemistry 99(8) (1995) 1049–1056.
E.J. Delgado R., A thermal engine driven by a thermokinetic oscillator, J. Phys. Chem. 100(26) (1996) 11144–11147.
L.K. Forbes, M.R. Myerscough and B.F. Gray, On the presence of limit-cycles in a model exothermic chemical reaction: Sal'nikov's oscillator with two temperature-dependent reaction rates, Proc. Roy. Soc. London Ser. A 435 (1991) 591–604.
B.F. Gray and L.K. Forbes, Analysis of chemical kinetic systems over the entire parameter space IV. The Sal'nikov oscillator with two temperature-dependent reaction rates, Proc. Roy. Soc. London Ser. A 444 (1994) 621–642.
M.J. Sexton and L.K. Forbes, An exothermic chemical reaction with linear feedback control, Dynamics and Stability of Systems 11(3) (1996) 219–238.
U.S. Herges and E.H. Twizell, A finite-difference method for the numerical solution of the Sal'nikov thermokinetic oscillator problem, Proc. Roy. Soc. London Ser. A 449 (1995) 255–271.
D.P. Coppersthwaite, J.F. Griffiths and B.F. Gray, Oscillations in the H2 +Cl2 reaction: experimental measurements and numerical simulation, J. Chem. Phys. 95(18) (1991) 6961–6967.
P. Gray and J. Griffiths, Thermokinetic combustion oscillations as an alternative to thermal explosion, Combustion and Flame 78 (1989) 87–98.
J.F. Griffiths, S.R. Kay and S.K. Scott, Oscillatory combustion in closed vessels: Theoretical foundations and their experimental verification, in: Twenty-Second International Symposium on Combustion(The Combustion Institute, 1988) pp. 1597–1607.
H.S. Sidhu, M.J. Sexton, M.I. Nelson, G.N. Mercer and R.O. Weber, A simple combustion process in a semibatch reactor, in: EMAC 2000 Proceedings, eds. R.L. May, G.F. Fitz-Gerald and I.H. Grundy (The Institution of Engineers, Australia, 2000) pp. 251–254.
M. Golubitsky and D. Schaeffer, Singularities and Groups in Bifurcation Theory, Applied Mathematical Sciences 51, Vol. 1, 1st ed. (Springer, New York, 1985).
M. Golubitsky and D. Schaeffer, A theory for imperfect bifurcation theory via singularity theory, Comm. Pure Appl. Math. 32 (1979) 21–98.
V. Balakotaiah and D. Luss, Analysis of the multiplicity patterns of a CSTR, Chem. Engrg. Commun. 13 (1981) 111–132.
V. Balakotaiah and D. Luss, Analysis of the multiplicity patterns of a CSTR, Chem. Engrg. Commun. 19 (1982) 185–189.
V. Balakotaiah and D. Luss, Structure of the steady-state solutions of lumped-parameter chemically reacting systems, Chem. Engrg. Sci. 37(11) (1982) 1611–1623.
V. Balakotaiah and D. Luss, Global analysis of the multiplicity features of multi-reaction lumped parameter systems, Chem. Engrg. Sci. 39(5) (1984) 865–881.
M. Golubitsky and D. Schaeffer, The classification theorem, in: Singularities and Groups in Bifurcation Theory, Vol. 1, 1st ed. (Springer, New York, 1985) chapter IV, paragraph 2 pp. 196–202.
V. Balakotaiah, Steady-state multiplicity features of open chemically reacting systems, in: Reacting Flows: Combustion and Chemical Reactors, Part II, ed. G.S.S. Ludford, Lectures in Applied Mathematics, Vol. 24 (American Mathematical Society, Providence, RI, 1986) pp. 129–161.
P.L. Simon, H. Farkas and M. Wittman, Constructing global bifurcation diagrams by the parametric representation method, J. Comput. Appl. Math. 108 (1999) 157–176.
E.J. Doedel, T.F. Fairgrieve, B. Sandstede, A.R. Champneys, Y.A. Kuznetsov and X.Wang, AUTO 97: Continuation and bifurcation software for Ordinary Differential Equations (with HomCont) (March 1998). Available by anonymous ftp from ftp.cs.concordia.ca/pub/doedel/auto.
D. Drysdale, An Introduction to Fire Dynamics, 2nd ed. (Wiley, New York, 1999).
R.M. Chemburkar, M. Morbidelli and A. Varma, Parametric sensitivity of a CSTR, Chem. Engrg. Sci. 41(6) (1986) 1647–1654.
H.F. Coward and G.W. Jones, Limits of flammability of gases and vapours, in: Bulletin(United States Bureau of Mines, 1952) p. 503.
R. Hirst, N. Savage and K. Booth, Measurement of inerting concentrations, Fire Safety J. 4 (1981/82) 47–168.
D.B. Spalding, A theory of inflammability limits and flame-quenching, Proc. Roy. Soc. London Ser. A 240 (1957) 83–100.
H.S. Sidhu, M.I. Nelson, G.N. Mercer and R.O.Weber, Dynamical analysis of an elementary X+Y? P reaction in a continuously stirred tank reactor, J. Math. Chem. 28(4) (2000) 353–375.
M.I. Nelson, Flammability limits in batch reactor: a Semenov model, submitted.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nelson, M., Sidhu, H. Bifurcation Phenomena for an Oxidation Reaction in a Continuously Stirred Tank Reactor I. Adiabatic Operation. Journal of Mathematical Chemistry 31, 155–186 (2002). https://doi.org/10.1023/A:1016222831327
Issue Date:
DOI: https://doi.org/10.1023/A:1016222831327