Abstract
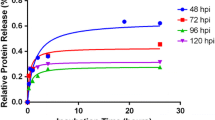
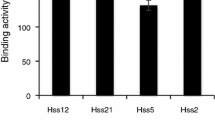
The proteolytic activity of High-Five insect cell culture supernatants was analysed using substrate gel electrophoresis (zymography). During growth in serum-free media, High-Five cells constitutively expressed and secreted proteases that were active on casein gel but not on gelatin or bovine serum albumin gels. Two main protease bands were visible at about 41–42 kDa and 32–33 kDa. By addition of various protease inhibitors in the incubation buffer, the proteases were identified as metalloproteases as complete and specific inhibition of the proteolytic activities was only obtained by 1,10-phenanthroline.
Similar content being viewed by others
References
Agathos SN (1996) Insect cell bioreactors. Cytotechnology 20: 173–189.
Barrett AJ, Rawlings ND (1991) Proteinases. Biochem. Soc. Trans. 19: 707–715.
Barrett AJ, Rawlings ND, Woessner JF (1998) Handbook of Proteolytic Enzymes. San Diego: Academic Press.
Cruz PE, Martins PC, Alves PM, Peixoto CC, Santos H, Moreira JL, Carrondo MJT (1999) Proteolytic activity in infected and noninfected insect cells: degradation of HIV-1 Pr55gag particles. Biotechnol. Bioeng. 65: 133–143.
Davis TR, Wickham TJ, McKenna KA, Granados RR, Shuler ML, Wood HA (1993) Comparative recombinant protein production of eight insect cell lines. In Vitro Cell. Dev. Biol. 29: 388–390.
Gotoh T, Miyazaki Y, Kikuchi K, Bentley WE (2001a) Investigation of sequential behavior of carboxyl protease and cysteine protease activities in virus-infected Sf-9 insect cell culture by inhibition assay. Appl. Microbiol. Biotechnol. 56: 742–749.
Gotoh T, Miyazaki Y, Sato W, Kikuchi KI, Bentley WE (2001b) Proteolytic activity and recombinant protein production in virusinfected Sf-9 insect cell cultures supplemented with carboxyl and cysteine protease inhibitors. J. Biosci. Bioeng. 92: 248–255.
Grosch H-W, Hasilik A (1998) Protection of proteolysis-prone recombinant proteins in baculovirus expression systems. BioTechniques 24: 930–934.
Ikonomou L, Bastin G, Schneider Y-J, Agathos SN (2001) Design of an efficient medium for insect cell growth and recombinant protein production. In Vitro Cell. Dev. Biol. Anim. 37: 549–559.
Lefebvre V, Peeters-Joris C, Vaes G (1991) Production of gelatindegrading matrix metalloproteinases ('type IV collagenases') and inhibitors by articular chondrocytes during their dedifferentiation by serial subcultures and under stimulation by interleukin-1 and tumor necrosis factor alpha. Biochim. Biophys. Acta 1094: 8–18.
Naggie S, Bentley WE (1998) Appearance of protease activities coincides with p10 and polyhedrin-driven protein production in the baculovirus expression system: effects on yield. Biotechnol. Prog. 14: 227–232.
Naggie S, Hu Y-C, Pulliam-Holoman TR, Bentley WE (1997) Substrate (gelatin) gel electrophoretic method for analysis of protease activity in insect (Sf-9) cells. Biotechnol. Tech. 11: 297–300.
Palomares LA, Ramirez OT (1998) Insect cell culture: recent advances, bioengineering challenges and implications in protein production. In: Galindo E, Ramirez OT, eds. Advances in Bioprocess Engineering II. Dordrecht: Kluwer Academic Publishers, pp. 25–52.
Pham M-Q, Naggie S, Wier M, Cha HJ, Bentley WE (1999) Human interleukin-2 production in insect (Trichoplusia ni) larvae: effects and partial control of proteolysis. Biotechnol. Bioeng. 62: 175–182.
Pyle LE, Barton P, Fujiwara Y, Mitchell A, Fidge N (1995) Secretion of biologically active human proapolipoprotein A-I in a baculovirus-insect cell system: protection from degradation by protease inhibitors. J. Lipid Res. 36: 2355–2361.
Reuveny S, Kemp CW, Eppstein L, Shiloach J (1992) Carbohydrate metabolism in insect cell cultures during cell growth and recombinant protein production. Ann. N.Y. Acad. Sci. 665: 230–237.
Salvesen G, Nagase H (1994) Inhibition of proteolytic enzymes. In: Beynon RJ, Bond JS, eds. Proteolytic Enzymes: A Practical Approach. Oxford: IRL Press, pp. 83–104.
Slack JM, Kuzio J, Faulkner P (1995) Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J. Gen. Virol. 76: 1091–1098.
Wang M-Y, Pulliam TR, Valle M, Vakharia VN, Bentley WE (1996) Kinetic analysis of alkaline protease activity, recombinant protein production and metabolites for infected insect (Sf9) cells under different DO levels. J. Biotechnol. 46: 243–254.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ikonomou, L., Peeters-Joris, C., Schneider, YJ. et al. Supernatant proteolytic activities of High-Five insect cells grown in serum-free culture. Biotechnology Letters 24, 965–969 (2002). https://doi.org/10.1023/A:1015692323167
Issue Date:
DOI: https://doi.org/10.1023/A:1015692323167