Abstract
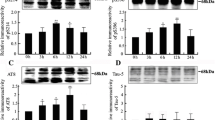
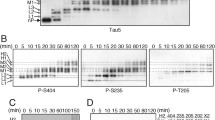
Alzheimer disease and related dementia are characterized by the presence of hyperphosphorylated tau aggregated into filaments. The role of tau phosphorylation in the fibrillogenesis has not yet been unraveled. Therefore, it is important to know which phosphatases can dephosphorylate tau protein in vivo. The effect of recombinant purified calcineurin (CN(PP2B)) and several calcineurin mutants on tau phosphorylation was studied in two neuronal like cell lines PC12 and SH-SY5Y. The modulation of tau phosphorylation at Ser199/Ser202, Ser396/Ser404, Ser262/Ser356, and Thr181 sites was examined in these cell lines using the phosphorylation state-dependent antitau antibodies Tau 1, PHF1, 12E8, and AT270. The results have shown that CN directly dephosphorylates all of those sites of tau protein. Recombinant calcineurin introduced into cells that have previously been treated with okadaic acid and cyclosporin A, which are inhibitors of phosphatases (PP1/PP2A and PP2B), has a direct effect on the phosphorylation status on all phosphorylation sites studied. We conclude that calcineurin is (besides PP2A) a important modulator of tau phosphorylation in vivo.
Similar content being viewed by others
REFERENCES
Biernat, J., Gustke, N., and Drewes, G. (1993). Phosphorylation of ser 263 strongly reduces binding of tau to microtubules: Distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11:153–163.
Borasio, G. D., John, J., Wittinghofer, A., Barde, Y.-A., Sendner, M., and Heumann, R. (1989). Ras P21 protein promotes suvival and fiber out growth of cultured embryonic neurons. Neuron 2:1087–1
Bradford, M. M. (1976).Arapid and sensitive method for the quantity of microgram quantities of protein dye binding. Anal. Biochem. 72:48–54.
Davis, P. K., Dudek, S. M., and Johnson, G. V. W. (1997). Select alterations in protein kinases and phosphatases during apoptosis of differentiated PC12 cells. J. Neurochem. 68:2338–2347.
Drewes, G., Ebneth, A., Preuss, U., Mandelknw, E. M., and Mandelkow, E. (1997). MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89:297–308.
Garver, T. D., Kincaid, R. L., Conn, R. A., and Billingsley, M. L. (1999). Reduction of calcineurin activitu in brain by antisense oligonucleotides leads to persistent phosphorylation of J protein at Thr181 and Thr231. Mol. Phamacol. 55:632–641.
Grundke-Iqbal, I., Iqbal, K., Quinlan, M., Tung, Y.-C., Zaidi, M. S., and Wisniewski, H. M. (1986). Microtubule-associated protein tau: A component of Alzheimer paired helical filaments. J. Biol. Chem. 261:6084–6089.
Iqbal, K., Alonso, A. C., Gondal, J. A., Gong, C. X., Haque, N., Khatoon, S., Sengupta, A., Wang, J. Z., and Grundke-Iqbal, I. (1999). Inhibition of neurofibrillary degeneration: A rational and promising therapeutic target. In Iqbal, K., Sweab, D. F.,Winblad, B., and Wisniewski, H. M. (eds.), Alzheimer's Disease and Related Disorders,Wiley, New York, pp. 643–654.
Kawashima, M. M., Hasegawa, M., Takio, K. Suzuki, M., Yoshida, H., Watanobe, A., Titani, K., and Ihara, Y. (1995). Hyperphosphorylation of tau in PHF. Neurobiol. Aging 16:265–380.
Kayyali, U. S., Zhang, W., Yee, A. G., Seidman, J. G., and Potter, H. (1997). Cytoskeletal changes in the brains of mice lacking calcineurin A ?. J. Neurochem. 68:1668–1678.
Klee, C.B., Ren, H., and Wang, X.T. (1998). Regulation of the calmodulin-stimulated protein phosphotase, calcineurin. J. Biol. Chem. 273:13367–13370.
Ladner, C. J., Czech, J., Maurice, J., Lorens, S. A., and Lee, J. M. (1996). Reduction of calcineurin enzymatic activity in Alzheimer's disease-correlation with neuropathologic changes. J. Neuropathol. Exp. Neurol. 55:924–931.
Liu, J., Farmer, J. D., Lane, W. S., Friedman, J., Weissman, I., and Schrelber, S. L. (1991). Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807–815.
Lovestone, S., and Reynolds, C. H. (1997). The phosphorylation of tau: A critical stage in neurodevelopment and neurodegenerative processes. Neuroscience 78:309–324.
Mandelkow, E., Song, Y.-H., Schweers, O., Marx, A., and Mandelkow, M. (1995). On the structure of microtubules, tau, and paired helical filaments. Neurobiol. Aging 16:347–353.
Ortiz,D., Baldwin, M. M., and Lucas, J. J. (1987). Transient correction of genetic defects in cultured animal cells by introduction of functional proteins. Mol. Cell. Biol. 7:3012–3017.
Shibasaki, F., Price, E. R., Milan, D., and Mckeen, F. (1996). Role of kinases and the phosphatase calcineurin in the nuclear shutting of transcription factor NF-AT4. Nature 382:370–373.
Spillantin, M. G., and Goedert, M. (1998). Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 21:428–433.
Tanaka, T., Zhong, T., Iqbal, K., Trenkner, E., and Grundke-Iqbal, I. (1998). The regulation of phosphorylation of Óin SY5Y neuroblastoma cells: The role of protein phosphatases. FEBS Lett. 426:248–254.
Wei,Q., and Lee, E.Y.C. (1997a). Expression and reconstitution of calcineurinAandBsubunits. Biochem. Mol. Biol. Int. 41:169–177.
Wei, Q., and Lee, E. Y. C. (1997b). Mutagenesis of loop connecting β strands 12 and 13 of calcineurin: Evidence for a strctural role in activity changes. Biochemistry 36:7418–7424.
Xie, H. Q., and Lohnson, G. V. W. (1998). Calcineurin inhibition prevents calpain-mediated proteolysis of tau in differentiated PC12 cells. J. Neurosci. Res. 53:153–164.
Yan, L. J., and Wei,Q. (1999). High activity of the calcineurinAsubunit with aV314 deletion. Biol. Chem. 380:1281–1285.
Yang, S. J., Zhang, L., and Wei, Q. (2000). Activities and properties of calcineurin catalytic domain. Chin. Sci. Bull. 45:1394–1398.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wei, Q., Holzer, M., Brueckner, M.K. et al. Dephosphorylation of Tau Protein by Calcineurin Triturated into Neural Living Cells. Cell Mol Neurobiol 22, 13–24 (2002). https://doi.org/10.1023/A:1015385527187
Issue Date:
DOI: https://doi.org/10.1023/A:1015385527187