Abstract
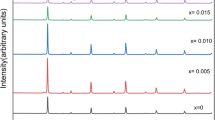
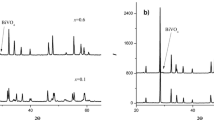
The mechanochemical reactions induced in 2CdO(HgO) + Sb2O5(Sb2O3) oxide mixtures by high-energy ball milling were studied by x-ray diffraction. New pyrochlore and fluorite phases were found to be formed at a high rate. Using thermal analysis, the temperature ranges of the structural and chemical transformations in the reaction products were determined and their compositions were refined. The synthesized materials are characterized by disordered structures, intermediate valence states, high vacancy concentrations, and deficit of the harder component, in line with the earlier proposed model for the reaction zone. The relatively high thermal stability of the metastable compounds suggests that mechanochemical ceramic processing can be used to fabricate unique ceramic materials and nanocomposites.
Similar content being viewed by others
REFERENCES
Zyryanov, V.V., Sysoev, V.F., and Boldyrev, V.V., Mechanochemical Ceramic Processing, Dokl. Akad. Nauk SSSR, 1988, vol. 300, no. 1, pp. 162–165.
Zyryanov, V.V., Mechanochemical Ceramic Processing: Developments and Prospects, Mekhanokhimicheskii sintez v neorganicheskoi khimii (Mechanochemical Synthesis in Inorganic Chemistry), Avvakumov, E.G., Ed., Novosibirsk: Nauka, 1991, pp. 102–125.
Zyryanov, V.V., Mechanochemical Effects in Oxide Systems, Extended Abstract of Doctoral (Chem.) Dissertation, Novosibirsk: Inst. of Mechano-and Solid-State Chemistry, Siberian Div., Russ. Acad. Sci., 2000.
Zyryanov, V.V., A Model for the Reaction Zone in Powders Mechanically Activated in a Planetary Mill, Neorg. Mater., 1998, vol. 34, no. 12, pp. 1525–1534 [Inorg. Mater. (Engl. Transl.), vol. 34, no. 12, pp. 1290-1298].
Butyagin, P.Yu., On the Critical State of Substances in Mechanochemical Transformations, Dokl. Akad. Nauk, 1993, vol. 331, no. 3, pp. 311–314.
Zyryanov, V.V., Mechanochemical Synthesis of Lead Titanate, Neorg. Mater., 1999, vol. 35, no. 9, pp. 1101–1107 [Inorg. Mater. (Engl. Transl.), vol. 35, no. 9, pp. 935-940].
Avvakumov, E.G., Rykov, A.I., Pavlyukhin, Yu.T., and Kormilitsina, Z.A., Mechanochemical Synthesis of BaPbxBi1-x O3 Solid Solutions, Izv. Akad. Nauk SSSR, Neorg. Mater., 1990, vol. 26, no. 8, pp. 1712–1714.
Kosova, N.V., Devyatkina, E.T., Avvakumov, E.G., et al., Mechanochemical Synthesis of Dicalcium Ferrite with the Perovskite Structure, Neorg. Mater., 1998, vol. 34, no. 4, pp. 478–485 [Inorg. Mater. (Engl. Transl.), vol. 34, no. 4, pp. 385-390].
Isupova, L.A., Sadykov, V.A., Avvakumov, E.G., and Kosova, N.V., Mechanochemical Activation and Technology of High-Temperature Oxide Catalysts, Khim. Interesakh Ustoich. Razvit., 1998, vol. 6, no. 2/3, pp. 207–211.
Karagedov, G.R. and Konovalova, E.A., Mechanochemical Activation of Mixtures of Metal Oxides with Close Anion Packing, Materialy nauchno-tekhnicheskogo seminara SNG (Proc. CIS Sci. Technol. Conf.), Mogilev, 1992, p. 44.
Sepelak, V., Steinike, U., Tkacova, K., et al., Disordering Structure and Properties of Mechanically Synthesized Spinel Ferrites, Khim. Interesakh Ustoich. Razvit., 1998, vol. 6, no. 2/3, pp. 183–188.
Karagedov, G.R., Konovalova, E.A., and Lyakhov, N.Z., Mechanochemical Synthesis of Lithium Ferrites, Sib. Khim. Zh., 1993, vol. 1, pp. 108–114.
Heinike, G., Tribochemistry, Berlin: Akademie, 1984. Translated under the title Tribokhimiya, Moscow: Mir, 1987.
Zyryanov, V.V. and Laz'ko, F.A., Mechanochemical Synthesis of PbMoO4, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1990, no. 2, pp. 96–100.
Zyryanov, V.V., Mechanochemical Synthesis of MM'O4 Oxides with the Scheelite Structure, Neorg. Mater., 2000, vol. 36, no. 1, pp. 70–73 [Inorg. Mater. (Engl. Transl.), vol. 36, no. 1, pp. 54-59].
Avvakumov, E.G., Potkin, A.R., and Samarin, O.I., USSR Inventor's Certificate no. 975 068, Otkrytiya, Izobret., 1982, no. 43.
Zyryanov, V.V., Sysoev, V.F., Boldyrev, V.V., and Korosteleva, T.V., USSR Inventor's Certificate no. 1375328, Otkrytiya, Izobret., 1988, no. 7, p. 39.
Fleischer, M., Wilcox, R.E., and Matzko, J.J., Microscopic Determination of Nonopaque Minerals, US Geol. Surv. Bull., 1984, no. 1627. Translated under the title Mikroskopicheskoe opredelenie prozrachnykh mineralov, Leningrad: Nedra, 1987.
Zyryanov, V.V., Lyakhov, N.Z., and Boldyrev, V.V., ESR Study of Titania Mechanolysis, Dokl. Akad. Nauk SSSR, 1981, vol. 258, no. 2, pp. 394–397.
Zyryanov, V.V., Electron Magnetic Resonance in Weakly Magnetic Dielectrics Processed by Mechanical Impulses, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1988, vol. 6, no. 19, pp. 9–13.
Zyryanov, V.V., ESR and EFR in the Weak Magnetic Insulators Treated by Mechanical Pulses, XXIV Congress AMPERE: Magnetic Resonance and Related Phenomena, Pozna, 1988, B-109.
Krotova, N.A., Linke, E., and Khrustalev, Yu.A., Fast-Electron Emission Induced by Disintegration of Ionic Crystals, Dokl. Akad. Nauk SSSR, 1973, vol. 208, no. 1, pp. 138–141.
Pervukhina, N.V., Magarill, S.A., Borisov, S.V., et al., Crystal Chemistry of Low-Valent Mercury Compounds, Usp. Khim., 1999, vol. 68, no. 8, pp. 683–707.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zyryanov, V.V. Structure of Mechanochemical Reaction Products in the Systems CdO(HgO)–Sb2O5(Sb2O3). Inorganic Materials 37, 1138–1148 (2001). https://doi.org/10.1023/A:1012545009010
Issue Date:
DOI: https://doi.org/10.1023/A:1012545009010