Abstract
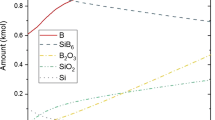
The standard molar enthalpy of formation Δf H m 0=–760±12 kJ for amorphous silicon nitride a-Si3N4 has been determined from fluorine combustion calorimetry measurements of the massic energy of the reaction: a-Si3N4(s)+6F2(g)=3SiF4(g)+2N2(g). This value combined with Δf H m 0= –828.9±3.4 kJ for a-Si3N4 indicates that determined for the first time molar enthalpy change for the transition from amorphous to α-crystalline form Δtrs H m 0=69±13 kJ is very evident, in spite of its large uncertainty range resulting from impurity corrections.
Similar content being viewed by others
References
Sainte-Claire Deville and H. Wöhler, F. Liebigs Ann. Chem., 110 (1859) 248.
Silicon nitride in microelectronics and solar cells, in Gmelin Handbook of Inorganic and Organometallic Chemistry, Suppl. Vol. B 5c, Ed. Springer, Berlin 1991.
Non-electronic applications of silicon nitride, in Gmelin Handbook of Inorganic and Organometallic Chemistry, Suppl. Vol. B 5e, Ed. Springer, Berlin 1994.
R. Riedel and M. Seher, J. Eur. Ceram. Soc., 7 (1991) 21.
L. V. Gurvitch, I. V. Veyts and C. B. Alcock, Thermodynamic Properties of Individual Substances, Vol. 2. Hemisphere, New York 1991.
P. A. G. O'Hare, I. Tomaszkiewicz, Ch. M. Beck and H.-J. Seifert, J. Chem. Thermodyn., 31 (1999) 303.
P. A. G. O'Hare, I. Tomaszkiewicz and H.-J. Seifert, J. Mater. Res., 12 (1997) 3203.
Reactant silicon nitride characteristics, in Gmelin Handbook of Inorganic and OrganometallicChemistry, Suppl. Vol. B 5d1, Ed. Springer, Berlin 1995, p. 36.
P. A. G. O'Hare and G. A. Hope, J. Chem. Thermodyn., 24 (1992) 639.
W. N. Hubbard, Experimental Thermochemistry. Vol. II, Ed. H. A. Skinner, Interscience, New York 1962.
P. A. G. O'Hare, J. Chem. Thermodyn., 17 (1985) 349.
D. White, J.-H. Hu and H. L. Johnston, J. Chem. Phys., 21 (1953) 1149.
S. D. Hamann, W. J. McManamey and J. F. Pearse, Trans. Faraday. Soc., 49 (1953) 351.
A. Heintz and R. N. Lichtenthaler, Ber. Bunsenges. Phys. Chem., 80 (1976) 962.
G. K. Johnson, J. Chem. Thermodyn., 18 (1986) 801.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tomaszkiewicz, I. Thermodynamics of Silicon Nitride. Standard molar enthalpy of formation of amorphous Si3N4 at 298.15 K. Journal of Thermal Analysis and Calorimetry 65, 425–433 (2001). https://doi.org/10.1023/A:1012472801483
Issue Date:
DOI: https://doi.org/10.1023/A:1012472801483