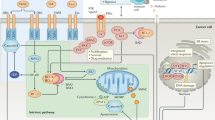
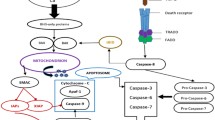
Abstract
Apoptosis is the primary means by which most radio- and chemotherapy modalities kill cancer cells, and abnormalities in the apoptotic pathways may contribute to disease pathogenesis of cancer. Multiple Myeloma (MM) is a hematological malignancy which will affect 14,000 new individuals in the United States in 2001 and remains irreversibly fatal despite all available therapies. The current review focuses on the studies of apoptotic and survival signaling pathways in MM cells, which have both identified novel apoptotic and anti-apoptotic proteins and provided targets for novel therapeutics.
Similar content being viewed by others
References
Kerr JF, Willie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Kerr JF, Winterford CM, Harmon BV. Apoptosis-its significance in cancer and cancer therapy. Cancer 1994; 73: 2013–2026.
Willie AH, Kerr JF, Currie AR. Cell death: The significance of apoptosis. Int Rev Cytol 1980; 68: 251–306.
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456–1462.
Urashima M, Chauhan D, Hatziyanni M, et al. CD40 ligand triggers interleukin-6 mediated B cell differentiation. Leukemia Research 1996; 20: 507–515.
Anderson KC, Jones RC, Morimoto C, Leavitt P, Barut B. Response of purified myeloma cells to hematopoietic growth factors. Blood 1989; 73: 1915–1924.
Uchiyama H, Barut BA, MohrbacherAF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates IL-6 secretion. Blood 1993; 82: 3712–3720.
Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kB. Blood 1996; 87:1104–1112.
Urashima M, Ogata A, Chauhan D, et al. Transforming growth factor b1: Differential effects on multiple myeloma versus normal B cells. Blood 1996; 87: 1928–1938.
Anderson K. Advances in the biology of multiple myeloma: Therapeutic applications. Semin Oncol 1999; 26: 10–22.
Bataille R, Jourdan M, Zhang XG, et al. Serum levels of interleukin-6, a potent myeloma cell growth factor, as a reflection of disease severity in plasma cell dyscrasias. Journal of Clinical Investigation 1989; 84: 2008–2011.
Chauhan D, Pandey P, Ogata A, et al. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene 1997; 15: 837–843.
Chauhan D, Hideshima T, Pandey P, et al. RAFTK/PYK2-dependent and independent apoptosis in multiple myeloma cells. Oncogene 1999; 18: 6733–6740.
Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol 1995; 162: 248–255.
Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochemical Journal 1998; 334: 297–314.
Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell 1994; 76: 253.
Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 1995; 86: 1243.
Ogata A, Chauhan D, Teoh G, et al. Interleukin-6 triggers cell growth via the ras-dependent mitogen-activated protein kinase cascade. J Immunology 1997; 159: 2212–2221.
Ogata A, Chauhan D, Urashima M, Teoh G, Treon SP, Anderson KC. Blockade of mitogen-activated protein kinase cascade signaling in interleukin-6 independent multiple myeloma cells. Clinical Cancer Research 1997; 3: 1017–1022.
Feng G, Hui C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science (Wash. DC) 1993; 259: 1607–1611.
Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science 1993; 259: 1611–1614.
Jernberg-Wiiklund H, Petterson M, Nilsson K. Recombinant interferon-gamma inhibits the growth of IL-6 dependent human multiple myeloma cell lines in vitro. European Journal of Hematology 1991; 46: 231–239.
Portier M, Zhang XG, Caron E, Lu ZY, Bataille R, Klein B. Gamma-interferon in multiple myeloma: Inhibition of interleukin 6 dependent myeloma cell growth and downregulation of IL-6 receptor expression in vitro. Blood 1993; 81: 3076–3082.
Ogata A, Nishimoto N, Shima Y, Yoshizaki K, Kishimoto T. Inhibitory effect of All-trans retinoic acid on the growth of freshly isolated myeloma cells via interference with interleukin-6 signal transduction. Blood 1994; 84: 3040–3046.
Schwabe M, Brini AT, Bosco MC, et al. Disruption by interferon-gamma of autocrine IL-6 growth loop in IL-6 dependent U266 myeloma cells by homologous and heterologous downregulation of the IL-6 receptor alpha and beta chains. J Clin Invest 1994; 94: 2317–2325.
Chauhan D, Hideshima T, Treon SP, et al. Functional interaction between retinoblastoma protein and stress activated protein kinase in multiple myeloma cells. Cancer Res 1999; 59: 1192–1195.
Meydan N, Grunberger T, Dadi H, et al. Inhibition of acute lymphoblastic leukaemia by JAK-2 inhibitor. Nature 1996; 379: 645–648.
Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996; 2: 561–566.
Marra F, Choudhary GG, Abboud HE. Interferon-gamma mediated activation of STAT-1 regulates growth-induced mitogenesis. J Clin Invest 1996; 98: 1218–1239.
Frank DA. STAT signaling in the pathogenesis and treatment of cancer. Mol Med 1999; 5: 432–456.
Carlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of STA-3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999; 10: 105–115.
Neiborowska-Skaska M, Wasik MA, Slupianek A, et al. Siganal tranducer and activator of transcription (STAT)-5 activation by Bcr-Abl is dependent on intact Src homology (SH3) and SH2 domains of Bcr-Abl, and is required for leukemogenesis, J Exp Med 1999; 189: 1229–1242.
Takeda K, Noguchi K, Shi W, et al. Targeted disruption of the mouse STAT-3 gene leads to early embryonic lethality. Proc Natl Acad Sci 1997; 94: 3801–3804.
Urashima M, Teoh G, Ogata A, et al. Characterization of p16INK4A expression in multiple myeloma and plasma cell leukemia. Clinical Cancer Research 1997; 3: 2173–2179.
Teoh G, Urashima M, Ogata A, et al. MDM2 protein overexpression promotes proliferation and survival of multiple myeloma cells. Blood 1997; 90: 1982–1992.
Urashima M, Ogata A, Chauhan D, et al. Interleukin-6 promotes multiple myeloma cell growth via phosphorylation of Retinoblastoma protein. Blood 1996; 88: 2219–2227.
Urashima M, Teoh G, Chauhan D, et al. Interleukin-6 overcomes p21WAF1upregulation and G1 growth arrest induced by dexamethasone and interferon-g in multiple myeloma cells. Blood 1997; 90: 279–289.
Urashima M, Teoh G, Ogata A, et al. Role of CDK4 and p16INK4A in interleukin-6-mediated growth of multiple myeloma. Leukemia 1997; 11: 1957–1963.
Chauhan D, Kharbanda S, Ogata A, et al. Interleukin-6 inhibits Fas-induced apoptosis and stress-activated protein kinase activation in multiple myeloma cells. Blood 1997; 89: 227–234.
Chauhan D, Pandey P, Ogata A, et al. Cytochrome-c dependent and independent induction of apoptosis in multiple myeloma cells. Journal of Biological Chemistry 1997; 272: 29995–29997.
Xu F, Sharma S, Gardner S, et al. Interleukin-6 induced inhibition of multiple myeloma cell apoptosis: Support for the hypothesis that protection is mediated via inhibition of the JNK/SAPK pathway. Blood 1998; 92: 241–251.
Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science 1998; 281: 1312–1316.
Casiola-Rosen L, Nikolson DW, Chang T, et al. Apopain/ CPP32 cleaves proteins that are essential for cellular repair-A fundamental principle of apoptotic death. J Exp Med 1996;183: 1957–1964.
Emoto Y, Mannome Y, Meinhardt G, et al. Proteolytic activation of protein kinase C-Delta by an ICE-Like protease in apototic cells. Embo J 1995; 14: 6148–6156.
Santos AS, Naoufal Z, Kroemer G. Mitochondria as regulator of apoptosis: Doubt no more. Biochimica et Biophysica Acta 1998; 1366: 151–165.
Marchetti P, Zamzami N, Joseph B, et al. The novel retiniod 6-[3-(1-adamantyl]-2naphtalene carboxylic acid can trigger apoptosis through a mitochondrial pathway independent of the nucleus. Cancer Research 1999; 59: 6257–6266.
Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to triggerDNA fragmentation during apoptosis. Cell 1997; 89: 175–184.
Yang J, Liu X, Balla K, et al. Prevention of apoptosis by Bcl-2-Release of Cytochrome-c from the mitochondria blocked. Science 1997; 275: 1129–1136.
Kluck RM, Kashibatta S. A lively meeting of a deathly topic. Apoptosis 1997; 2: 337–342.
Avraham S, London R, Fu YG, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. Journal of Biological Chemistry 1995; 270: 27742–27751.
Lev S, Moreno H, Martinez R, et al. Protein tyrosine kinase PYK2 involved in CA2+-induced regulation of ion channel and MAP kinase functions. Nature 1995; 376: 737–745.
Xiong WC, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. Journal of Cell Biology 1997; 139: 529–539.
Feng GS. Shp-2 tyrosine phosphatase: Signaling one cell or many. Experimental Cell Research 1999; 253: 47–54.
Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Current Opinion in Cell Biology 1997; 9: 193–204.
Streuli M. Protein tyrosine phosphatases in signaling. Current Opinion in Cell Biology 1996; 8: 182–188.
Vanvactor D, O'Reilly AM, Neel BG. Genetic analysis of protein tyrosine phosphatases. Current Opinion in Genetics & Development 1998; 8: 112–126.
Saxton TM, Pawson T. Morophogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proceedings of the National Academy of Sciences of the United States of America 1999; 96: 3790–3795.
Shi ZQ, Lu W, Feng GS. The SHP-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-jun NH2-terminal mitogen-activated protein kinases. Journal of Biological Chemistry 1998; 273: 4904–4908.
Marengere LEM, Waterhouse P, Duncan GS, et al. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science 1996; 272: 1170–1173.
Treon SP, Teoh G, Urashima M, et al. Anti-estrogens induce apoptosis of multiple myeloma cells. Blood 1998; 92: 1749–1757.
Treon SP, Chauhan D, Raje N, Teoh G, Webb I, Anderson KC. Recombinant human TNF-related apoptosis inducing ligand (TRAIL) induces apoptosis of human multiple myeloma cells. Blood 1998; 92: 6349.
Vacca A, Ribatti D, Presta M, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999; 93: 3064–3073.
Munshi N, Wilson CC, Penn MJ, et al. Angiogenesis in newly diagnosed multiple myeloma: Poor prognosis with increased microvessel density in bone marrow biopsies. Blood 1998; 92 (Suppl): 98a.
Rajkumar SV, Fonseca R, Witzig TE, Gertz MA, Greipp PR. Bone marrow angiogenesis in patients achieving complete response after stem cell transplantation for multiple myeloma. Leukemia 1999; 13: 469–472.
Singhal S, Mehta J, Desikan R, et al. Anti-tumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571.
Weber DM, Gavino M, Delasalle K, Rankin K, Girault S, Alexanian R. Thalidomide alone or with dexamethasone for multiple myeloma. Blood Supple 1999; 94: Abstract 2686.
Geitz H, Handt S, Zwingengerger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology 1996; 32: 213–221.
Corral LG, Haslett PAJ, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-a. J Immunol 1999; 163: 380–386.
Haslett PAJ, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 1998; 187: 1885–1892.
Bellamy W, Richter L, Frutiger Y, Grogan T. Expression of vascular endothelial growth factor and its receptor in hematological malignancies. Cancer Research 1999; 59: 728–733.
Teicher BA, Ara G, Herbst R, Palonmbella VJ, Adams J. The Proteosome inhibitor PS-341 in cancer therapy. Clin Can Res 1999; 5: 2638–2645.
Sonneveld P. Drug resistance in myeloma. In: VI International Workshop on Multiple Myeloma. Boston, 1997.
Salmon SE, Dalton WS, Grogan TM, et al. Multidrug-resistant myeloma: Laboratory and clinical effects of verapamil as a chemosensitizer. Blood 1991; 78: 44–50.
Reed JC. Bcl-2 family of proteins. Oncogene 1998; 17: 3225–3236.
Reed JC. Dysregulation of apoptosis in cancer. J Clin Onco 1999; 17: 2941–2948.
Campos L, Sabido O, Roualt JP, Guyatat D. Effects of Bcl-2 antisense oligodeoxenucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood 1994; 84: 595–600.
Feinman R, Koury J, Thames M, Barlogie B, Epstein J. Role of NF-kB in the rescue of multiple myeloma cells from glucocorticoids-induced apoptosis by Bcl-2. Blood 1999; 93: 3044–3052.
Bloem A, Lockhorst H. Bcl-2 antisense therapy in multiple myeloma. Pathologie et Biologie 1999; 47: 216–220.
Tosi P, Pellacani A, Visani G, et al. In vitro treatment with retinoids decreases Bcl-2 protein expression and enhances dexamethasone-induced cytotoxicity and apoptosis in multiple myeloma cells. Euro J Hemat 1999; 62: 143–148.
Tu YP, Renner S, Xu FH, et al. Bcl-x expression in multiple myeloma-possible indicator of chemoresistance. Can Res 1998; 58: 256–262.
Puthier D, Bataille R, Barille S, et al. Myeloma cell growth arrest, apoptosis, and interleukin 6 receptor modulation induced by EB1089, a vitamin D3 derivative, alone or in association with dexamethasone. Blood 1996; 88: 4659–4666.
Schwarze MMK, Hawley RG. Prevention of myeloma cell apoptosis by ectopic bcl-2 expression or interleukin-6 mediated upregulation of bcl-XL. Cancer Res 1995; 55: 2262.
Sumatran VN, Ealovega MW, Nunez G, Clarke MF, Wicha MS. Overexpression of Bcl-x(s), sensitizes MCF-7 cells to chemotherapy-induced apoptosis. Can Res R 1995; 55: 2507–2510.
Clarke MF, Apel IJ, Benedict MA, et al. A recombinant Bclx( s) adenovirus selectively induces apoptosis in cancer cells but not in normal bone marrow cells. Proc Natl Acad Sci 1995; 92: 11024–11028.
Tai YT, Strobel T, Kufe D, Cannistra SA. In vivo cytotoxicity of ovarian cancer cells throuh tumor-selective expression of the BAX gene. Can Res 1999; 59: 2121–2126.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chauhan, D., Anderson, K.C. Apoptosis in multiple myeloma: Therapeutic implications. Apoptosis 6, 47–55 (2001). https://doi.org/10.1023/A:1009620027205
Issue Date:
DOI: https://doi.org/10.1023/A:1009620027205