Abstract
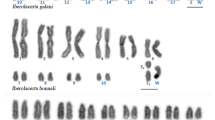
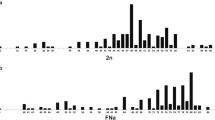
A multiple Robertsonian fission chromosomal race of the Liolaemus monticola complex in Chile is described and is shown to be the most derived and the most complex among the Liolaemus examined thus far. The 29 karyotyped lizards analysed from the locality of Mina Hierro Viejo, Petorca, Provincia de ValparaUso, Chile, exhibited a diploid chromosomal number ranging from 42 to 44, and several polymorphisms. The polymorphisms included: a pair 1 fission; a pair 2 fission plus a pericentric inversion in one of the fission products, which moved the NOR and satellite from the tip of the long arm of the metacentric 2 to the short arm of the fission product; a fission in pair 3; a polymorphism for an enlarged chromosome pair 6; and a polymorphism for a pericentric inversion in pair 7. This population is fixed for a fission of chromosome pair 4. A total of 76% of the lizards analysed were polymorphic for one or more pairs of chromosomes. We have compared these data with other Liolaemus monticola chromosomal races and calculated the Hardy–Weinberg ratios for the polymorphic chromosome pairs in this Multiple-Fission race. Karyotypic differences between the Northern (2n = 38–40) and the Multiple-Fission (2n = 42–44) races were attributed mainly to Robertsonian fissions, an enlarged chromosome and pericentric inversions involving the macrochromosomes and one microchromosome pair.
Similar content being viewed by others
References
Arévalo EC, Porter CA, González A, Mendoza F, Camarillo JL, Sites JW JR (1991) Population cytogenetics and evolution of the Sceloporus grammicus complex (Iguanidae) in Central Mexico. Herpetol Monogr 5: 79–115.
Cole CJ (1970) Karyotypes and evolution of the spinolosus group of lizards in the genus Sceloporus. Am Mus Novitates 2431: 1–47.
Donoso-Barros R (1966) Reptiles de Chile. Santiago, Chile: Ediciones de la Universidad de Chile.
Espinoza ND, Formas RD (1976) Karyological pattern of two Chilean lizard species of the genus Liolaemus (Sauria: Iguanidae). Experientia 32: 299–301.
Evans EP, Breckon G, Ford CE (1964) An air-drying method for meiotic preparations from mammalian testes. Cytogenetics 3: 289–294.
Frost DR, Etheridge R (1989) A phylogenetic analysis and taxonomy of iguanian lizards (Reptilia: Squamata). Misc Pub Mus Nat Hist (University of Kansas) 81: 1–65.
Gorman GC (1973) The chromosomes of reptilia, a cytotaxonomic interpretation. In: Chiarelli AB, Capanna E, eds. Cytotaxonomic and Vertebrate Evolution. New York: Academic Press. pp. 349–417.
Goyenechea I, Mendoza-Quijano F, Flores-Villela O, Reed KM (1996) Extreme chromosomal polytypy in a population of Sceloporus grammicus (Sauria: Phrynosomatidae) at Santuario Mapethé, Hidalgo, México. J Herpetol 30: 39–46.
Hall WP (1973) Comparative population cytogenetics, speciation and evolution of the iguanid genus Sceloporus. Ph. D. Dissertation, Harvard University, Cambridge, Massachusetts, USA.
Hall WP (1983) Mode of speciation and evolution in the sceloporine iguanid lizards. I. Epistemology of the comparative approach and introduction to the problem. In: Rhodin AGJ, Miyata K, eds. Advances in Herpetology and Evolutionary Biology. Cambridge, Massachusetts: Mus Comp Zool. pp. 643–679.
Hall WP, Selander RK (1973) Hybridization of karyotypically differentiated populations in the Sceloporus gammicus complex (Iguanidae). Evolution 22: 226–242.
King M (1993) Species Evolution: the Role of Chromosome Change. London: Cambridge University Press. p. 336.
Lamborot M (1991) Karyotypic variation among populations of Liolaemus monticola (Tropiduridae) separated by riverine barriers in the Andean range. Copeia 1991: 1044–1059.
Lamborot M (1993) Chromosomal evolution and speciation in some chilean lizards. Evolución Biológica 7: 133–151.
Lamborot M, Alvarez-Sarret E (1989) Karyotypic characterization of some Liolaemus lizards in Chile (Iguanidae). Genome 32: 393–403.
Lamborot M, Alvarez-Sarret E (1993) Karyotypic variation within and between populations of the Liolaemus monitcola (Tropiduridae) separated by the Maipo River in the Coastal Range of Central Chile. Herpetologica 49: 435–449.
Lamborot M, Espinoza A, Alvarez E (1979) Karyotypic variation in Chilean lizards of the genus Liolaemus (Iguanidae). Experientia 35: 593–594.
Lamborot M, Alvarez E, Campos I, Espinoza A (1981) Karyotypic characterization of three chilean subspecies of Liolaemus monticola. J Hered 72: 328–334.
Müller von L, Hellmich W (1932) Beiträg zur Kenntnis der Herpetofauna Chiles. IV. Liolaemus monticola, ein weiterer neuer. RassenKreis aus den Hochanden Chiles. Zoolog Anzeiger Bd 99: 177–192.
Nevo E, Filippucci MG, Redi C, Korol A, Beiles A (1994) Chromosomal speciation and adaptative radiation of mole rats in Asia Minor correlated with increased ecological stress. Proc Natl Acad Sci USA 91: 8160–8164.
Paull D, Williams EE, Hall WP (1976) Lizard karyotypes from Galápagos Islands: chromosomes in phylogeny and evolution. Breviora Mus Comp Zool 441: 1–31.
Peters IA, Donoso-Barros R (1970) Catalogue of the Neotropical Squamata: Part II. Lizards and amphisbaenians. Bull US Nat Mus 297: 170–195.
Porter CA, Sites JW JR (1985) Normal disjunction in Robertsonian heterozygotes from a highly polymorphic lizard population. Cytogenet Cell Genet 39: 250–257.
Porter CA, Sites JW (1986) Evolution of Sceloporus grammicus complex (Sauria: Iguanidae) in Central Mexico: population cytogenetics. Syst Zool 35: 334–358.
Porter CA, Sites JW (1987) Evolution of Sceloporus grammicus complex (Sauria: Iguanidae) in Central Mexico. II. Studies on rates of nondisjunction and the occurrence of spontaneous chromosomal mutations. Genetica 75: 131–144.
Redi, CA, Capanna E (1988) Robertsonian heterozygotes in the house mouse and the fate of their germ cells. In: Daniel A, ed. The Cytogenetics of Mammalian Autosomal Rearrangements. New York: Alan R. Liss. pp. 315–359.
Shaw DD, Coates DJ, Arnold ML (1988) Complex patterns of chromosomal variation along a latitudinal cline in the grasshopper Caledia captiva. Genome 30: 108–117.
Sites JW Jr (1983) Chromosome evolution in the iguanid lizard Sceloporus grammicus. I. Chromosome polymorphisms. Evolution 37: 38–53.
Sites JW, Reed KM (1994) Chromosomal evolution, speciation, and systematics: some relevant issues. Herpetologica 50: 237–249.
Swofford DL, Selander RK (1989) Biosys-1: a computer program for the analysis of allelic variation in population genetics and biochemical systematics. Release 1.7. Illinois Natural History Survey, Champaign, IL.
Wallace B (1959) Influence of genetic systems on geographical distribution. Cold Spring Harbor Symp Quant Biol 24: 193–204.
White MJD (1973) Animal Cytology and Evolution. London: Cambridge University Press.
White MJD (1978a) Chain processes in chromosomal speciation. Syst Zool 27: 285–298.
White MJD (1978b) Modes of Speciation. San Francisco: WH Freeman.
Rights and permissions
About this article
Cite this article
Lamborot, M. A New Derived and Highly Polymorphic Chromosomal Race of Liolaemus Monticola (Iguanidae) from the 'Norte Chico' of Chile. Chromosome Res 6, 247–254 (1998). https://doi.org/10.1023/A:1009267821416
Issue Date:
DOI: https://doi.org/10.1023/A:1009267821416