Abstract
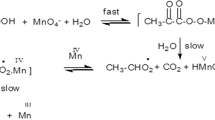
The kinetics of electron-transfer reactions between permanganate ion and ethyl and aryl methyl ketones have been studied in aqueous MeCO2H acid medium in the presence of HClO4 at different temperatures. For ethyl methyl ketone and XC6H4COMe (X = p-Cl, p-Br or p-NO2) the reaction obeys the rate law −d[MnO4−]/dt = (kKe[H+][MnO4−][RCO Me])/(1 + Ke[H+][RCOMe]).␣But the oxidations of XC6H4COMe (X = p-Me and p-OMe)␣follow the rate equation −d[MnO4−]/dt = k3[H+][MnO4−][RCOMe]. The reaction involves a fast pre-equilibrium with intermediate formation of a permanganate ester before the two-electron transfer, rate-determining, step. A number of thermodynamic parameters have been evaluated.
Similar content being viewed by others
References
R. Stewart in K. B. Wiberg (Ed.), Oxidation in Organic Chemistry, Academic Press, New York, 1965, Part A, (a) p. 35, (b) p. 25.
D. G. Lee, The Oxidation of Organic Compounds by Permanganate Ion and Hexavalent Chromium, Open Court, La Salle, IL. 1980.
K. B. Wiberg and R. Stewart, J. Am. Chem. Soc., 77, 1786 (1955).
R. Stewart and M. M. Mocek, Can. J. Chem., 41, 1160 (1963).
J. L. Kurz, J. Am. Chem. Soc., 85, 987 (1963).
J. L. Kurz, J. Am. Chem. Soc., 86, 2229 (1964).
F. Freeman, J. B. Brant, N. B. Hester, A. A. Kamego, M. L. Kasner, T. G. McLaughlin and E. W. Paul, J. Org. Chem., 35, 982 (1970).
F. Freeman, D. K. Lin and G. R. Moore, J. Org. Chem., 47, 56 (1982).
C. F. Koelsch, Org. Synth. 1955, Coll. Vol. III, 791.
A. Claus and W. Neukranz, J. Prakt. Chem. 44, 77 (1891).
K. K. Banerji, Z. Naturforsch., Teil B 27, 772 (1972).
K. K. Banerji, Bull. Chem. Soc. Jpn., 46, 3623 (1973).
Marigangaiah, P. Nath and K. K. Banerji, Aust. J. Chem., 29, 1939 (1976).
I. Bhatia and K. K. Banerji, J. Chem. Soc., Perkin Trans. 2, 1577 (1983).
R. M. Hasan, M. A. Mousa and M. H. Wahadan, J. Chem. Soc., Dalton Trans., 605 (1988).
J. F. Perez-Benito, R. M. Rodriguez, J. de Andres, E. Brillas and J. A. Garrido, Int. J. Chem. Kinet., 21, 71 (1989).
G. Sikkander, K. A. B. Ahamedi and S. Kannan, Inorg. Chem., 31, 845 (1992).
K. K. Sen Gupta, P. K. Sen and G. Mukhopadhyay, Transition Met. Chem., 18, 369 (1993).
P. K. Sen, A. Sanyal and K. K. Sen Gupta, Int. J. Chem. Kinet., 27, 379 (1995).
W. C. E. Higginson and D. Sutton, J. Chem. Soc., 1402 (1953).
Y. K. Gupta, J. Inorg. Nucl. Chem., 19, 179 (1961).
A. E. Cahill and H. Taube, J. Am. Chem. Soc., 74, 2312 (1952).
L. J. Kirschenbaum and J. R. Sutter, J. Phys. Chem., 70, 3863 (1966).
E. Zahonyi-Budo and L. I. Simandi, Inorg. Chim. Acta, 181, 149 (1991).
E. Zahonyi-Budo and L. I. Simandi, Inorg. Chim. Acta, 191, 1 (1992).
N. Bailey, A. Carrington, K. A. K. Lott and M. C. R. Symons, J. Chem. Soc., 290 (1960).
G. P. Panigrahi and B. P. Sahu, Int. J. Chem. Kinet., 25, 595 (1993).
F. R. Duke, J. Phys. Chem., 56, 882 (1952).
F. C. Tompkins, Trans. Faraday Soc., 39, 280 (1943).
L. I. Simandi and M. Jaky, J. Am. Chem. Soc., 98, 1995 (1976).
O. Exner, Nature (London), 201, 488 (1964).
H. T. Clarke, A Handbook of Organic Analysis, Edward Arnold, London, 1960, (a) p. 136, (b) p. 140, (c) and (d) p. 189, (e) p. 141.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sen, P.K., Mukhopadhyay, G. & Gupta, K.K.S. Kinetics and mechanism of the oxidation of alkyl and aryl methyl ketones by permanganate ion in aqueous ethanoic acid. Transition Metal Chemistry 23, 577–582 (1998). https://doi.org/10.1023/A:1006980502784
Issue Date:
DOI: https://doi.org/10.1023/A:1006980502784