Abstract
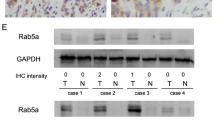
For the sake of better understanding the molecular mechanism of neoplasia, we have used the mRNA differential display technique to analyze two human lung adenocarcinoma cell lines, AGZY83-a and Anip973. Anip973 was isolated from AGZY83-a, but manifested much higher metastatic potential than the parent line. We found that a significant differential cDNA fragment in Anip973 was over-expressed, then over-expressed cDNA fragment was cloned and sequenced. It showed that the over-expressed cDNA in Anip973 was RAB5A cDNA. And the RAB5A cDNA sequence was corresponding between the two cells. To determine whether RAB5A may be differentially expressed in the two human lung adenocarcinoma cells at protein level, we further detected RAB5A protein in the two cells by using immunofluorescent method. RAB5A protein was upregulated in highly metastatic Anip973. We also detected the difference in RAB5A gene expression at RNA level in human non-small cell lung carcinoma by RT-PCR. Using immunohistochemical staining, we also examined RAB5A change at protein level in 45 cases human non-small cell lung carcinoma paraffin sections. The results proved the evidence of upregulation of RAB5A in malignant tumor, indicated over-expression of RAB5A gene was correlated with the malignant degree and metastatic potential of lung cancer(χ2 test, p <0.01). The RAB5A gene is a member of RAS superfamily, which can transcribe GTP-binding protein that plays an important role in signal transduction of protein trafficking at the cell surface and GDP/GTP cycle in the regulation of endocytotic membrane traffic. Thus our results indicated that over-expression of the RAB5A gene was involved in the process of transformation from AGZY83-a to the higher metastatic cell line Anip973. The result may be a powerful experimental evidence that over-expression of RAB5A gene associated with neoplasia metastasis.
Similar content being viewed by others
References
Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA.Molecular aspect of tumor cell invasion and metastasis. Cancer 1993; 71 (4): 1368–83.
Postel EH, Berberich SJ, Flint SJ, Ferrone CA. Human c-myc transcription factor PuF identified as nm23-H2 nucleoside diphosphate kinase, a candidate suppressor of tumor metastasis. Science 1993; 261: 478–80.
Steeg PS, Bevilacqua G, Kopper L, Thorgeirssoon UP, Talmadge JE, Liotta LA, Sobel M. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988; 80: 200–4.
Wang WR, Pan ZC, Yan XC, Liu X. Isolation of a metastatic associatic subpopulation from a human lung adenocarcinoma cell line in nude mice and studies on its biological properties. Chinese J Oncol 1987; 9 (6): 412–4.
Shi L, Qi Tengping, Wang WR. The selecting and maitainence of highly metastatic human cancer cell line in nude mice. J Pract Oncol 1991; 5(3): 29 (in Chinese).
Bao L, Loda M, Zetter BR. Thymosin β15 expression in tumor cell lines with varying metastatic potential. Clin Exp Metastasis 1998; 16 (3): 227–33.
Chishima T, Yang M, Miyagi Y, Li L, Tan Y, Baranov E, Shimada H, Moossa AR, Penman S, Hoffman M. Governing step of metastasis visualized in vitro 1997; 94: 11573–6.
Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992; 257 (8): 967–71.
Sun Y, Hegamyer G, Colburn NH. Molecular cloning of five messenger RNAs differentially express in preneoplastic or neoplastic JB6 mouse epidermal cells: one is homologous to human tissue inhibitor of metaloproteinase-3. Cancer Res 1994; 54(3): 1139–44.
Liang P, Averboukh L, Zhu WM, Pardee AB. RAS activation of genes: Mob-1 as a model. Proc Natl Acad Sci USA 1994; 91 (12): 12515–9.
Ito T, Kito K, Adati N, Mitsui Y, Hagiwara H, Sakaki Y. Fluorescent differential display: arbitrarily primed RT-PCR fingerprinting on an automated DNA sequencer. FEBS Letters 1994; 351: 231–6.
McClelland M, Mathieu-Daude F, Welsh J. RNA fingerprinting and differential display using arbitrarity primered PCR. TIG 1995; 11: 242–6.
Zahraour A, Touchot N, Chardin P, and Tavitian A. The Human Rab Gene5 encode a family of GTP-binding Proteins related to Yeast Ypt1 and SEC4 products involved in secretion. J Bio Chem 1989; 264 (21): 12394–401.
Gary MB, Channing JD. Emerging concepts in the ras superfamily of GTP-binding proteins. FASEB 1993; 7: 750–8.
Roya K-F and Channing JD. The ras signal transduction pathway. Cancer Metastasis Rev 1994; 13: 67–89.
Anne JR, Alan H. The small GTP-binding protein rho regulates the assembly of Focal adhesions and actin stressfibers in response to growth factors. Cell 1992; 70: 389–99.
Ando S, Karbuchi K, Sasaki T, Hiraoka K, Nishiyama T, Mizuno T, Asada M, Nunoi H, Matsuda I, Matsuura Y, Polakis P, McCormiok F, Takai Y. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J Bio Chem 1992; 267 (36): 25709–13.
Cchavrier P, Rarton RG, Houri HP, Simon SK, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and Endocytotic compartments. Cell 1990; 62: 317–29.
Buccl C, Parlon RG, Mather LH, Stunnenberg H, Simons K, Moflack B, and Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytotic pathway. Cell 1992; 70: 715–28.
Palade GE, Protein Kinasis: The dynamics of protein trafficking and stability. Cold Spring Harbor Symposia on Quantitative biology, Jan 1 1995; 60: 821–5.
Stenmark H, Valencia A, Martinez O, Ullrich O, Goud B, Zerial M. Distinct structural elements of Rab5 define its functional specificity. EMBO J 1994; 13: 575–83.
Simon I, Zerial M, Goody RS. Kinetics of interaction of Rab5 and Rab7 with nucleotides and magnesium ions. J Biol Chem 1996; 271: 20470–8.
Brechlker V, Chu WN, Baxter JD, Thibault G, Reudelhuber TL. A protease processing site is essential for protein sorting to the regulated secretory pathway. J Biol Chem 1996; 271: 20636–40, 1996.
Ruscianno D, Lorenzoni P, Burger MM. Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell density and is regulated by a cytotoxic tyrosine phosphatase activity. J Biol Chem 1996; 271: 20763–20769.
Aletta JM, Selbert MA, Nairn AC, Edelman AM. Activation of a calcium-calmodulin-dependent protein kinase I cascade in Pcl2 cells. J Bio Chem 1996; 271: 20930–20934.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yu, L., Hui-chen, F., Chen, Y. et al. Differential expression of RAB5A in human lung adenocarcinoma cells with different metastasis potential. Clin Exp Metastasis 17, 213–219 (1999). https://doi.org/10.1023/A:1006617016451
Issue Date:
DOI: https://doi.org/10.1023/A:1006617016451